43 energy diagram for hydrogen
Hydrogen molecule, energy level diagram - Big Chemical Encyclopedia Figure 9.1. Energy level diagram for hydrogen molecule, H2, and separated atoms H R = 00) and He R = 0). R = the Rydberg constant = 13.6057 eV = 0.5 a.u. (atomic unit of energy). Value from ionization potential of He (Is 2p P). Value from ionization potential of H2. › articles › s41560/018/0296-8Multi-site electrocatalysts for hydrogen evolution ... - Nature Dec 10, 2018 · where E[(X) *] denotes energy of X adsorbed on surface and E(H 2) is the energy of a hydrogen molecule in the gas phase. Binding energies of H, OH, and H 2 O are calculated as:
Draw a neat labelled energy level diagram of the Hydrogen atom. - Toppr Ask The energy gap between successive energy levels in a hydrogen atom: A rectangular corral of widths L x=L and L y=2L contains seven electrons. What multiple of 8mL 2h 2 gives the energy of (a) the first excited state, (b) the second excited state, and (c) the third excited state of the system of seven electrons? (d)Construct an energy-level diagram ...
Energy diagram for hydrogen
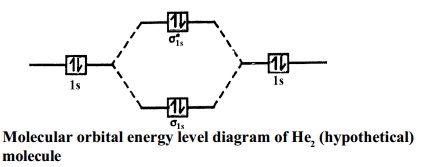
› hydrogen › renewable-electrolysisRenewable Electrolysis | Hydrogen and Fuel Cells | NREL This diagram depicts various scenarios for producing renewable hydrogen and electricity. Systems Integration and Component Development NREL develops power electronics interfaces for renewable electrolysis systems to characterize and test the performance of electrochemical devices. Energy level diagram for Molecular orbitals - Class Notes The first ten molecular orbitals may be arranged in order of energy as follow: σ (1s) <σ∗(1s) < σ (2s) <σ∗(2s) < π (2px) = π (2py) < σ (2pz) < π∗(2px) =π∗(2py) <π∗( 2pz) Relationship between electronic configuration and Molecular behaviour 1) Stability of molecules in terms of bonding and antibonding electrons en.wikipedia.org › wiki › Energy_densityEnergy density - Wikipedia This energy, E = mc 2, where m = ρV, ρ is the mass per unit volume, V is the volume of the mass itself and c is the speed of light. This energy, however, can be released only by the processes of nuclear fission (0.1%), nuclear fusion (1%), or the annihilation of some or all of the matter in the volume V by matter-antimatter collisions (100%).
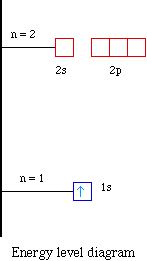
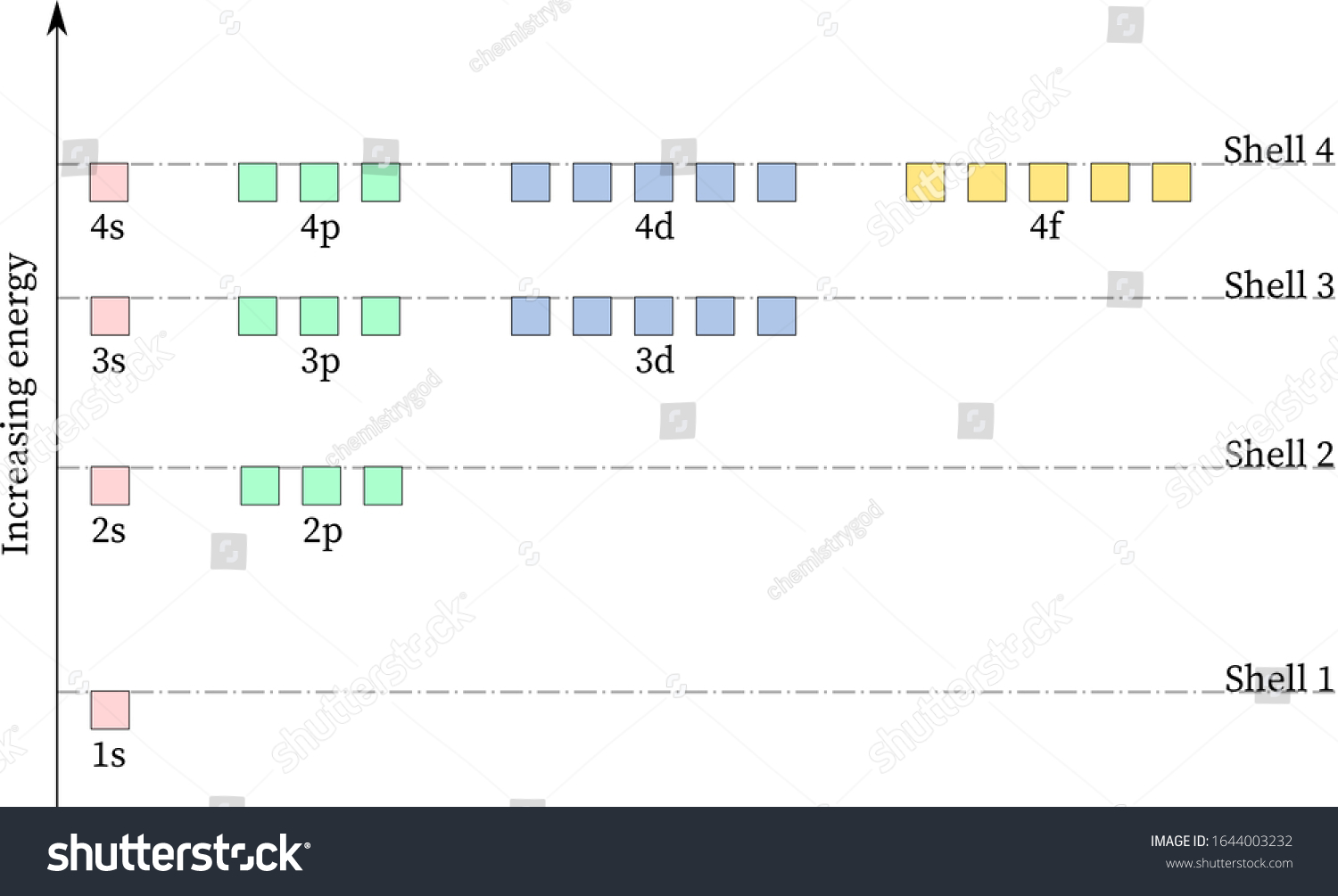
Energy diagram for hydrogen. › ecosystem › energy-flowEnergy Flow in an Ecosystem (With Diagram) - Biology Discussion Energy loss by respiration also progressively increases from lower to higher trophic states (Fig. 3.15). In the energy flow process, two things become obvious. Firstly there is one way along which energy moves i.e. unidirectional flow of energy. Energy comes in the ecosystem from outside source i.e. sun. Energy Level Diagram - Chemistry, Class 11, Structure of Atom Energies of orbitals of hydrogen and hydrogen like particles depend upon the value of principal quantum (n) number only , those of multi-electron atoms depend both upon principal quantum number( n ) as well as azimuthal quantum number(l). Diagram representing the arrangement of orbitals in order of their increasing energies are called energy level diagrams. […] Energy Level Diagram For Hydrogen - Mini Physics The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon. Energy Level Diagram - Different Energy Shells Around the Nucleus - BYJUS Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy levels is as follows:
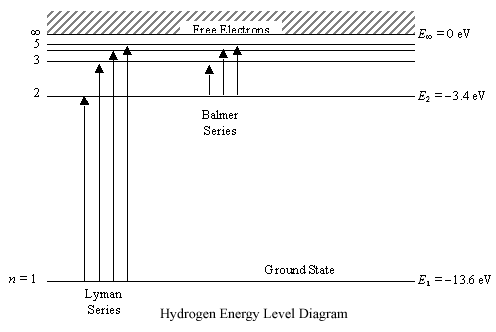
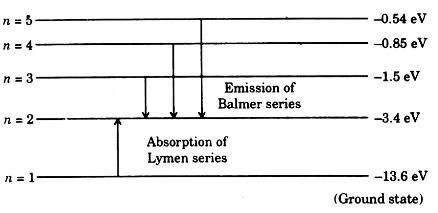
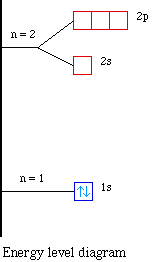
energyeducation.ca › encyclopedia › Nuclear_fusionNuclear fusion in the Sun - Energy Education The resulting energy is radiated out from the core of the Sun and moves across the solar system. It is important to note that the core is the only part of the Sun that produces any significant amount of heat through fusion (it contributes about 99%). The rest of the Sun is heated by energy transferred outward from the core. Steps Draw an energy level diagram for hydrogen atom. Indicate the ... The energy of the electron of the hydrogen atom in the nth orbit is En = - 13.6n^2 eV . Draw the energy level diagram for the hydrogen atom and show transitions corresponding to lives of Lyman and Balmer series. Class 12 >> Physics >> Atoms >> Bohr's Model >> The energy of the electron of the hydrog Question Energy Diagram for Hydrogen Atom - sliderbase.com Energy Diagram for Hydrogen Atom. The energy of a particular orbital is determined by its value of n. All orbitals with the same value of n have the same energy and are said to be degenerate. Hydrogen single electron occupy the lowest energy state, the ground state. If energy is put into the system, the electron can be transferred to higher ... Hydrogen(H) electron configuration and orbital diagram - Valenceelectrons Hydrogen orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction. This is clearly shown in the figure of the orbital diagram of hydrogen. Atomic Orbital Diagram for Hydrogen
atomic hydrogen emission spectrum - chemguide To find the normally quoted ionisation energy, we need to multiply this by the number of atoms in a mole of hydrogen atoms (the Avogadro constant) and then divide by 1000 to convert it into kilojoules. Note: It would be wrong to quote this to more than 3 significant figures. Hydrogen energies and spectrum - GSU At left is a hydrogen spectral tube excited by a 5000 volt transformer. The three prominent hydrogen lines are shown at the right of the image through a 600 lines/mm diffraction grating. An approximate classification of spectral colors: Violet (380-435nm) Blue(435-500 nm) Cyan (500-520 nm) Green (520-565 nm) Yellow (565- 590 nm) Orange (590-625 nm) Why do energy level diagrams differ for hydrogen and ... - Quora It really isn't the number of electrons that matter, it is the potential energy of the electrons and type of orbital that matter. You can actually use these energy level diagrams for helium, which (normally) has 2 electrons. What matters is that hydrogen and helium only have one orbital, which can very easily be described. The energy density of hydrogen: a unique property The energy density of hydrogen: a unique property Hydrogen has several remarkable properties. It stores enormous amounts of energy, has a boiling point as low as -252.9 °C, and differs significantly from most other fuels and energy carriers in terms of energy density. In this blog, we review the energy density of gaseous and liquid hydrogen.
The free energy diagram for hydrogen evolution reaction on different ... The free energy diagram for hydrogen evolution reaction on different coordinated Ni sites. The of an ideal catalyst for the HER should be close to 0 . Source publication Understanding the Activity...
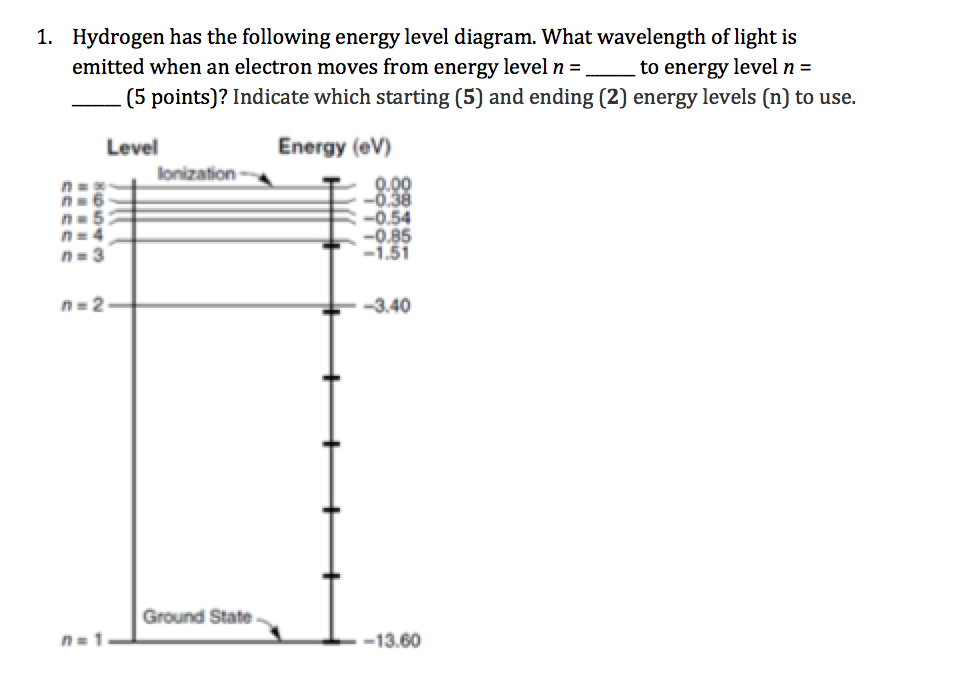
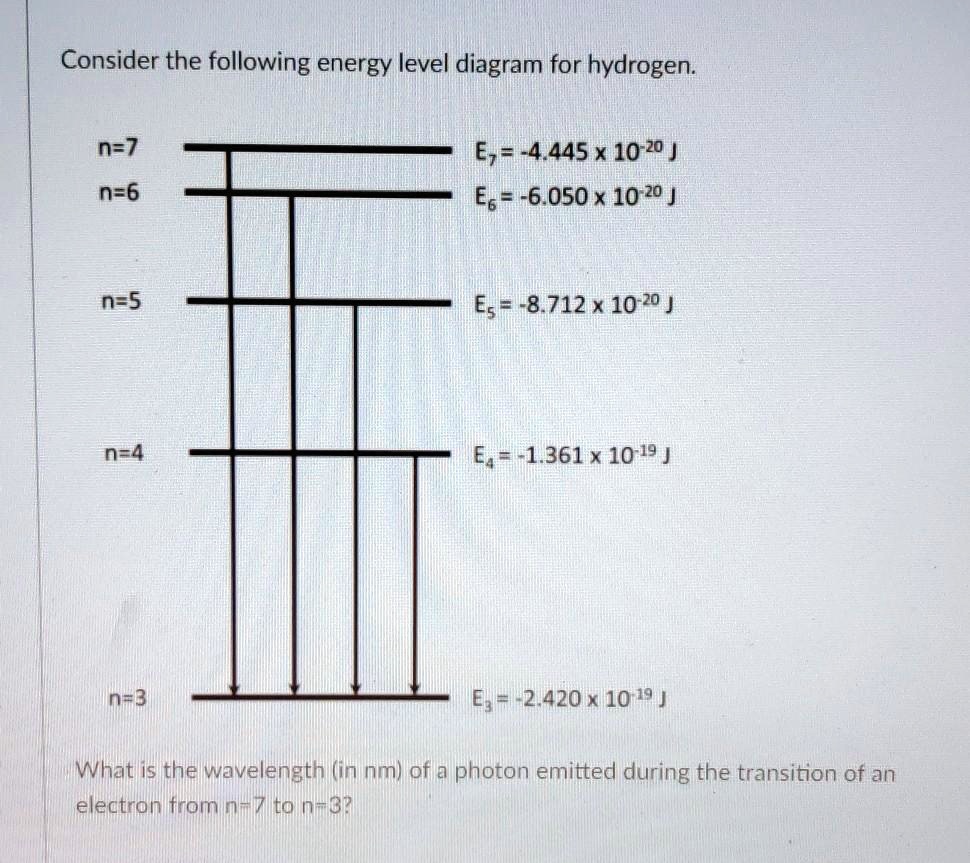
Partial Energy Level Diagram for Hydrogen - Course Hero View Lab Report - Partial Energy Level Diagram for Hydrogen from CHEM 2070 at Cornell University. Partial Energy Level Diagram for Hydrogen 1 = (6.626 x 10-37 KJ) (3.00 x 1017 nm/s) (6.022 x 1023
Energy level diagrams and the hydrogen atom - Boston University The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy.
Calculated free energy diagram for hydrogen evolution relative to the ... Therefore, the development of sustainable clean energy such as hydrogen is increasingly urgent due to the clean, efficient and renewable advantages [3,4].
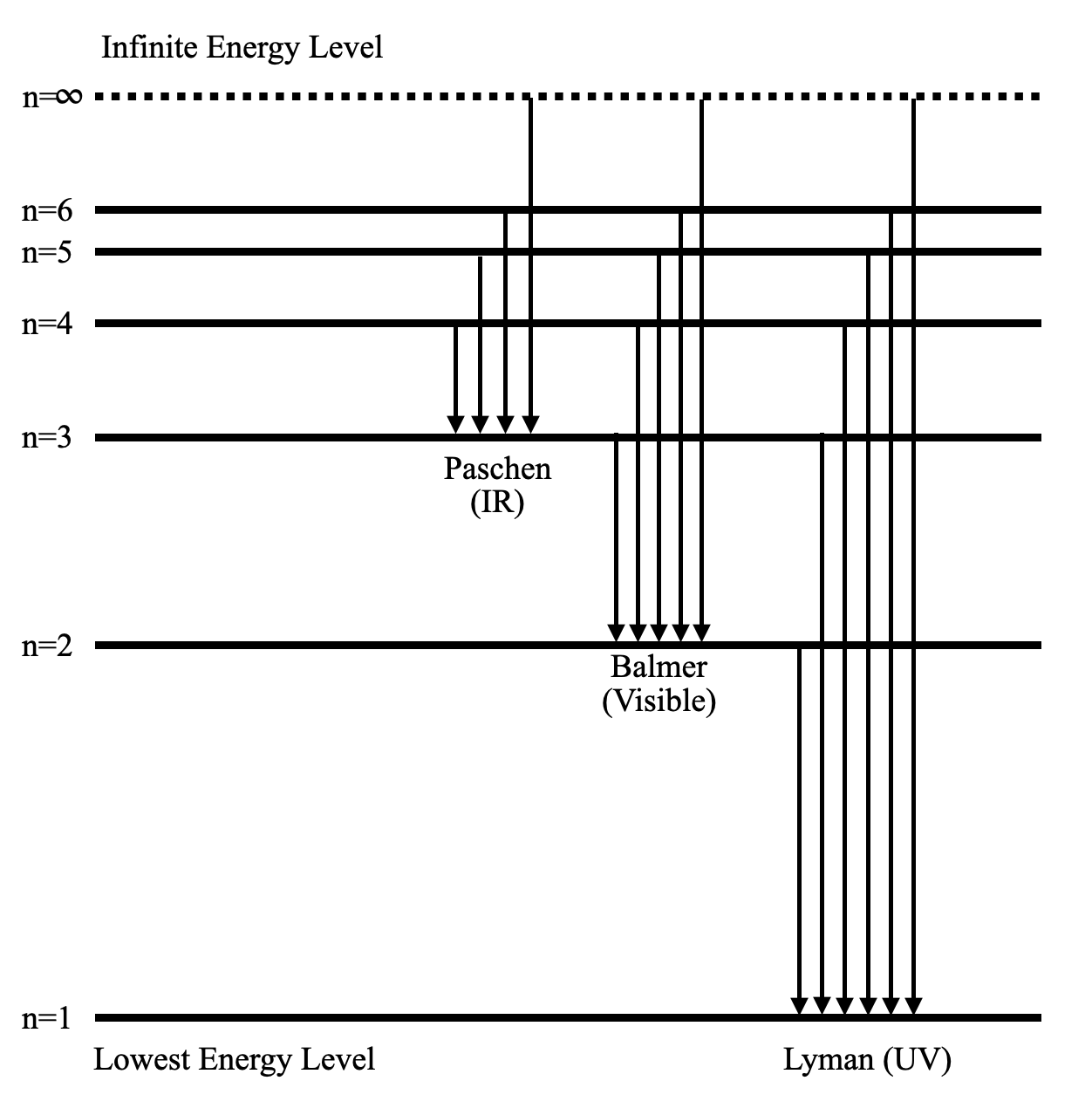
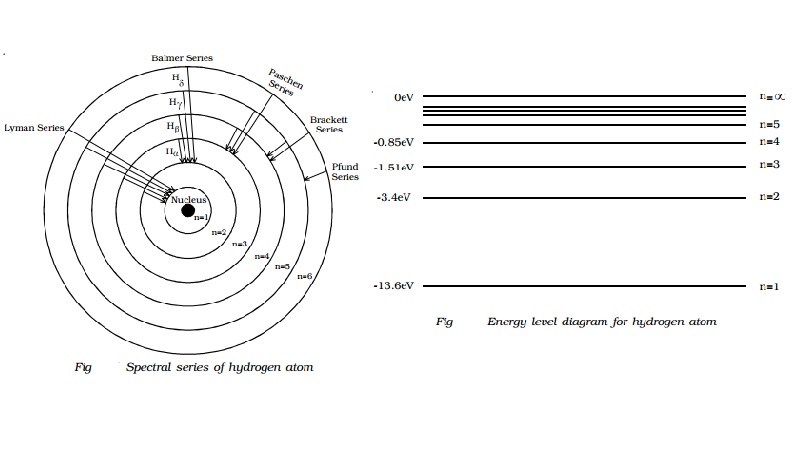
byjus.com › chemistry › hydrogen-spectrumHydrogen Spectrum - Balmer Series, Definition, Diagram, Spectrum The general formula for the hydrogen emission spectrum is given by: Where, n 1 = 1,2,3,4 … n 2 = n 1 +1. ν= wave number of electromagnetic radiation. The value 109,677 cm-1 is known as Rydberg constant for hydrogen. To learn more about hydrogen emission spectrum download BYJU'S - The Learning App. Read more: Emission spectrum and atomic spectra; Spectroscopy
Energy level diagrams and the hydrogen atom - Physics Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from ...
Energy-level diagram for the hydrogen atom - YouTube Here you will find curriculum-based, online educational resources for Chemistry for all grades. Subscribe and get access to thousands of top quality interact...
Hydrogen Fuel Basics | Department of Energy Hydrogen is an energy carrier that can be used to store, move, and deliver energy produced from other sources. Today, hydrogen fuel can be produced through several methods. The most common methods today are natural gas reforming (a thermal process), and electrolysis. Other methods include solar-driven and biological processes. Thermal Processes
Relativistic Energy Levels for Hydrogen Atom In nonrelativistic quantum mechanics, the energy levels of the hydrogen atom are given by the formula of Bohr and Schrödinger, , expressed in hartrees (assuming the appropriate correction for the reduced mass of the electron).The energy depends only on the principal quantum number and is -fold degenerate (including electron spin).In Dirac's relativistic theory, this degeneracy is partially ...
Hydrogen Production: Electrolysis | Department of Energy In many regions of the country, today's power grid is not ideal for providing the electricity required for electrolysis because of the greenhouse gases released and the amount of fuel required due to the low efficiency of the electricity generation process. Hydrogen production via electrolysis is being pursued for renewable (wind, solar, hydro, geothermal) and nuclear energy options.
Hydrogen molecule potential energy graph - Chemistry Stack Exchange Hydrogen molecule potential energy graph. In the above graph, I was confused at the point where the internuclear distance increases and potential energy become zero. Though internuclear distance is very small and potential energy has increased to zero.
OED: Hydrogen - in.gov Hydrogen may be used as a gas to generate electricity with natural gas power plants (either simple or combined-cycle) or to heat buildings similar to conventional natural gas. Hydrogen can be blended with natural gas resulting in cleaner energy without additional costs of new infrastructure. Energy storage. Researchers are also investigating ...
hydrogen - Sankey Diagrams The below Sankey diagram is shown on a webpage of HAW University of Applied Sciences. It shows the generation path of hydrogen from natural gas, and the overall energy yield. Unfortunately the diagram is too small to grasp the details. The Sankey arrows represent energy content. Losses are shown as black arrows.
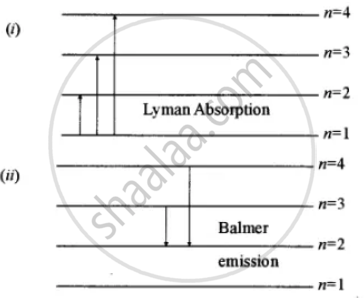
Diagram, Wavelength, Hydrogen Emission Spectrum - VEDANTU The Energy level diagram for the Hydrogen Atom indicates the different series of lines that is observed in the Spectrum. The horizontal lines witnessed refer to the different Energy levels. The vertical lines on the other hand refer to the transition that the Electron undergoes moving from the higher Energy level to the lower Energy level.
Draw Energy Level Diagram for Hydrogen Atom - Hyder Thatirly66 The solutions to the Schroedinger equation of such atoms are obtained by simply scaling the the solutions for the hydrogen cantlet. The energy levels scale with Z 2, i.due east. Due east n = -Z 2 *13.6 eV/n two. It takes more energy to remove an electron from the nucleus, because the bonny strength that must be overcome is stronger.
hydrogen atom energy level diagram - Energy Level Diagram hydrogen atom energy level diagram December 4, 2021 29 Views It has been said that hydrogen atoms are just as good as oxygen atoms, just as bright as the sun. They are also more efficient in converting carbon dioxide into energy and using them to make buildings and other things. However, a hydrogen atom's energy is not a perfect "energy level."
› hydrogen-sulfide-H2SHydrogen sulfide - Thermophysical Properties Hydrogen sulfide is used in the manufacture of chemicals, in metallurgy, and as an analytical reagent. The phase diagram of hydrogen sulfide is shown below the table. Chemical, physical and thermal properties of hydrogen sulfide: Values are given for gas phase at 25 o C /77 o F / 298 K and 1 bara, if not other phase, temperature or pressure given.
en.wikipedia.org › wiki › Energy_densityEnergy density - Wikipedia This energy, E = mc 2, where m = ρV, ρ is the mass per unit volume, V is the volume of the mass itself and c is the speed of light. This energy, however, can be released only by the processes of nuclear fission (0.1%), nuclear fusion (1%), or the annihilation of some or all of the matter in the volume V by matter-antimatter collisions (100%).
Energy level diagram for Molecular orbitals - Class Notes The first ten molecular orbitals may be arranged in order of energy as follow: σ (1s) <σ∗(1s) < σ (2s) <σ∗(2s) < π (2px) = π (2py) < σ (2pz) < π∗(2px) =π∗(2py) <π∗( 2pz) Relationship between electronic configuration and Molecular behaviour 1) Stability of molecules in terms of bonding and antibonding electrons
› hydrogen › renewable-electrolysisRenewable Electrolysis | Hydrogen and Fuel Cells | NREL This diagram depicts various scenarios for producing renewable hydrogen and electricity. Systems Integration and Component Development NREL develops power electronics interfaces for renewable electrolysis systems to characterize and test the performance of electrochemical devices.






















0 Response to "43 energy diagram for hydrogen"
Post a Comment