39 adiabatic process pv diagram
When the process is isobaric? Explained by FAQ Blog On a p-V diagram, the process occurs along a horizontal line (called an isobar) that has the equation p = constant. For a fixed mass of gas at constant pressure, the volume is directly proportional to the Kelvin temperature. What is isobaric process example? Thermodynamic Processes: Isobaric, Isochoric, Isothermal & Adiabatic ... The thermodynamic process is where the movement of heat energy takes place either within an object/area or between objects/areas. Learn about the isobaric, isochoric, isothermal, and adiabatic ...
Can isothermal process be zero? Explained by FAQ Blog What is ∆ U in adiabatic process? According to the definition of an adiabatic process, ΔU=wad. o C undergoes a reversible adiabatic expansion from 200. L to 800. Isothermal process Thermodynamics - Work, Heat & Internal Energy, PV Diagrams. 17 related questions found.

Adiabatic process pv diagram
en.wikipedia.org › wiki › Isobaric_processIsobaric process - Wikipedia An isobaric process is shown on a P–V diagram as a straight horizontal line, connecting the initial and final thermostatic states. If the process moves towards the right, then it is an expansion. If the process moves towards the left, then it is a compression. Sign convention for work Thermodynamic: Laws of Thermodynamics, System Variables - Embibe Adiabatic Process. Adiabatic process is a thermodynamic process in which there is no exchange of heat between the system and the surrounding from the initial to the final state. Since the heat exchange is zero \(∆Q = \rm{const} = 0\) Internal energy of the system is given by, \(∆U = nC_v ∆T\) Equation of thermodynamic process is given by, Adiabatic Compression: What Is, Working, Examples And … The work done in an adiabatic process can be derived from the formula for adiabatic process. PV ꝩ = Constant (K). This formula can be rewritten as P=KV-ꝩ In order to calculate the work done in adiabatic process , let us consider the system is compressed from the initial position of P1, V1 and T1 to final position P2, V2 and T2. The work ...
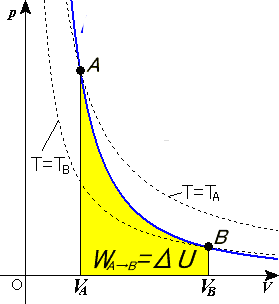
Adiabatic process pv diagram. Otto Cycle: Process, PV Diagram, Efficiency with Derivation ... Otto Cycle: Process, PV Diagram, Efficiency with Derivation & Applications [Explanation & PDF] ... Process 3-4: Reversible Adiabatic Expansion or Isentropic Expansion; Process 4-1: Constant Volume Heat Rejection; The explanation of above processes are as follows. Process 1-2: Reversible Adiabatic Compression or Isentropic Compression: The air is compressed into … In which process the PV indicator diagram is a straight line parallel ... In which process the PV indicator diagram is a straight line parallel to volume axis. by author. Q: In which process the PV indicator diagram is a straight line parallel to volume axis. (a) Isothermal. (b) Isobaric. (c) Irreversible. (d) Adiabatic. Click to See Answer : What is Otto Cycle - Complete Explaintion on P-v & T-s Diagram Pressure-Volume (p-v) Diagram of Four-stroke Otto cycle Engine The ideal Otto cycle consists of two constant volume and two reversible adiabatic or isentropic processes as shown on PV and T-S diagrams. Let the engine cylinder contains m kg of air at point 1. At this point, let p1, T1, andV1 be the pressure, temperature and volume of air. 1. What is meant by polytropic process? Explained by FAQ Blog A curve in a P-V diagram generated by the equation PV = const is called an isotherm. For an isothermal, reversible process, the work done by the gas is equal to the area under the relevant pressure -volume isotherm. It is given as WA→B=NkTlnVBVA W A → B = NkT ln V B V A . What is isothermal process give an example?
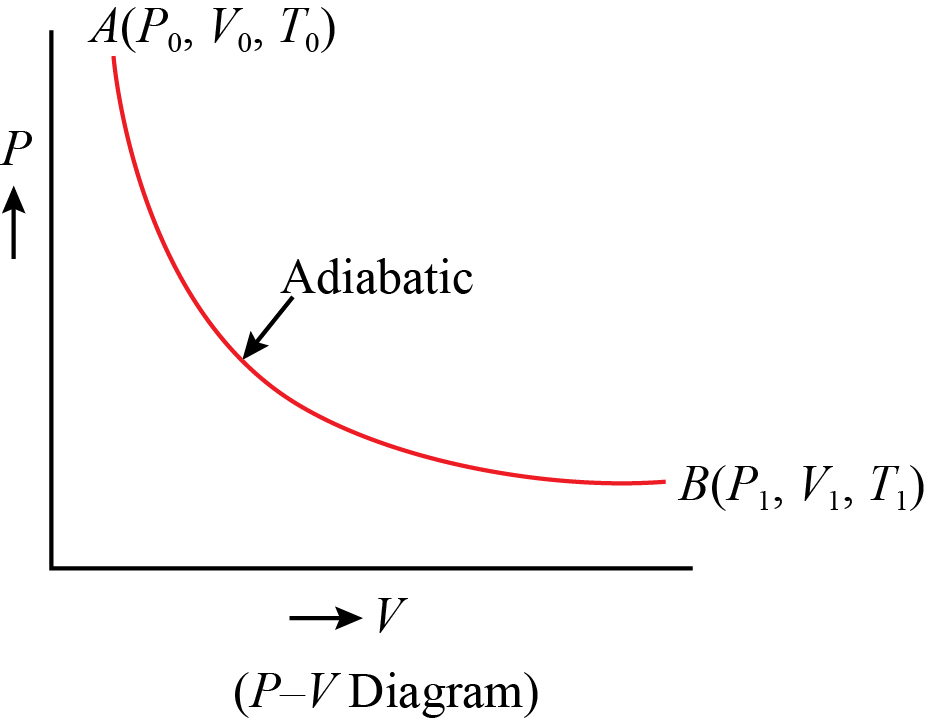
en.wikipedia.org › wiki › Pressure–volume_diagramPressure–volume diagram - Wikipedia A PV diagram plots the change in pressure P with respect to volume V for some process or processes. Typically in thermodynamics, the set of processes forms a cycle, so that upon completion of the cycle there has been no net change in state of the system; i.e. the device returns to the starting pressure and volume. [citation needed] What is Diesel Cycle - Processes with P-v and T-s Diagram Process 4-1 (Adiabatic Compression) The air is compressed adiabatically from temperature T4 to a temperature T1 represented by the graph 4-1 in Fig. In this process, no heat is absorbed or rejected by the air We see that the air has been brought back to its original conditions of pressure, volume and temperature, thus completing the cycle. Pv Diagram Calculator - icasmt.com Select the number of points in the cycle (3 or 4), and then choose which type of process.A PV diagram is a graph of Pressure as a function of Volume. There are four different situations that you can expect to see shown in PV diagrams: 1. • This formula would let us calculate the work done by the gas on the piston as it expands. Application of First Law to Non-flow Process » Gyan4all Adiabatic (or Isentropic) Process: In this process, no heat transfer takes place (Q = 0). Reversible adiabatic processes are frictionless and are called isentropic process. From First law of thermodynamics, Isentropic process. From gas equation, pv = m R T. Differentiating both sides, Pdv + vdp = m R dT.
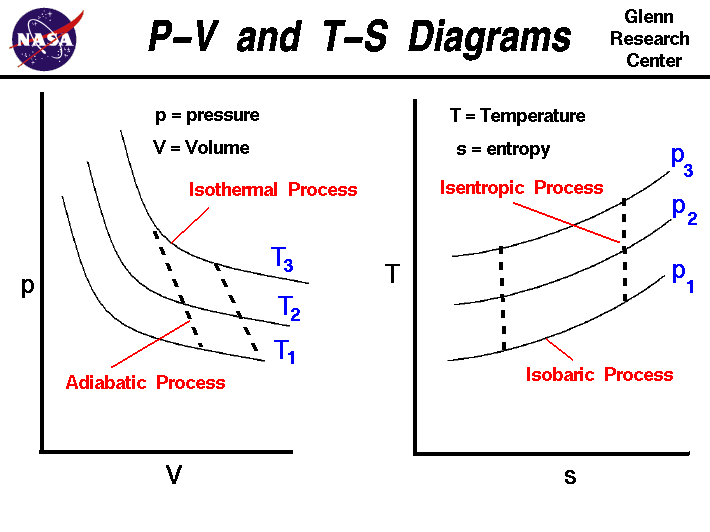
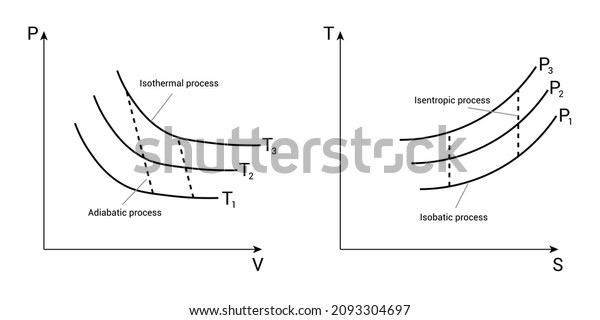
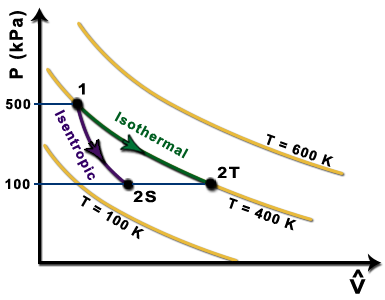
The area bounded by an isothermal and adiabatic curve in a PV diagram ... The area bounded by an isothermal and adiabatic curve in a PV diagram for heat engine represents : A. heat absorbed. B. heat reject. C. workdone. D. total kinetic energy. Answer: workdone. Heat MCQs. Thermodynamic process in which the change in volume of the system is zero tells that. If the volume of a given mass of a gas is doubled without ... What Is A Polytropic Process Used For? - On Secret Hunt Pressure-volume (P-V) and temperature-entropy (T-S) diagrams are often used as teaching. aids to describe refrigeration processes in introductory textbooks. They trace the path of a. hypothetical element of gas as it moves through a system during a complete thermodynamic. cycle. What is non quasi static process? › WWW › K-12P-V and T-S Diagrams - NASA May 13, 2021 · Lines of constant pressure curve from the lower left to upper right on a T-s diagram. A constant pressure process is called an isobaric process and this type of process occurs in the combustor of a gas turbine engine. During an isentropic process there is no change in the entropy of the system and the process is reversible. An isentropic ... What is Adiabatic Expansion – Adiabatic Compression - Thermal … 22.05.2019 · The adiabatic process can be expressed with the ideal gas law as: pV ... On a p-V diagram, the process occurs along a line (called an adiabat) that has the equation p = constant / V κ. For an ideal gas and a polytropic process, the case n = κ corresponds to an adiabatic process. Example of Adiabatic Expansion Assume an adiabatic expansion of helium (3 → 4) …
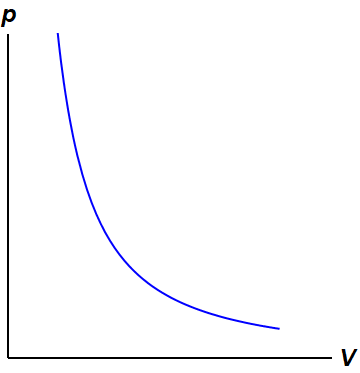
Thermodynamic Processes ~ ChemistryGod The isothermal process can be explained on a PV graph. In the above diagram, the system expands isothermally from state 1 (P1, V1) to state 2 (P2, V2). Assuming an ideal gas system, we can apply the ideal gas law, PV = nRT. And the equation of the isotherm becomes P = nRT/V.
Energy Balances — Introduction to Chemical and Biological … Important nomenclature. A closed system is one in which there is a fixed volume or space and no streams entering or leaving the system.. An open system is one in which there are streams entering and leaving. These streams can add or remove material and energy from a system. An adiabatic process is one in which there is no heat added or removed from the system.
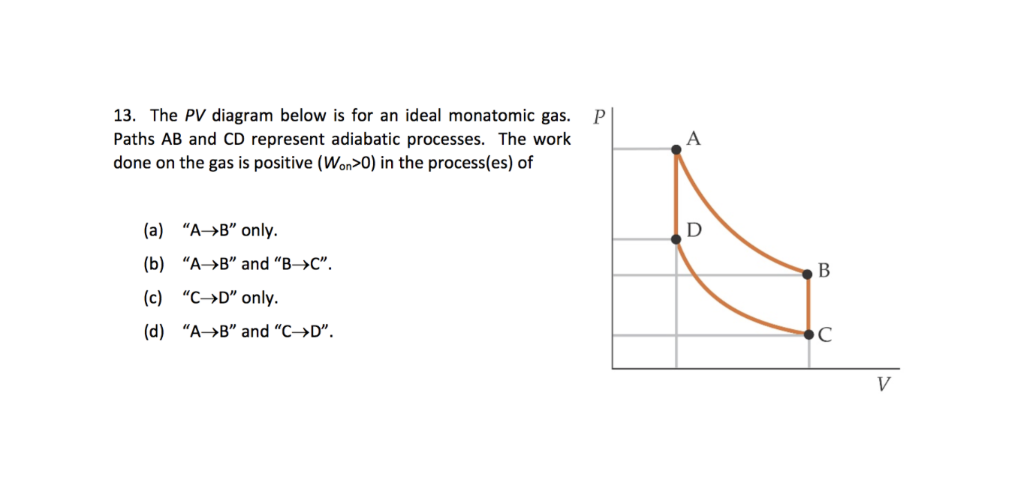
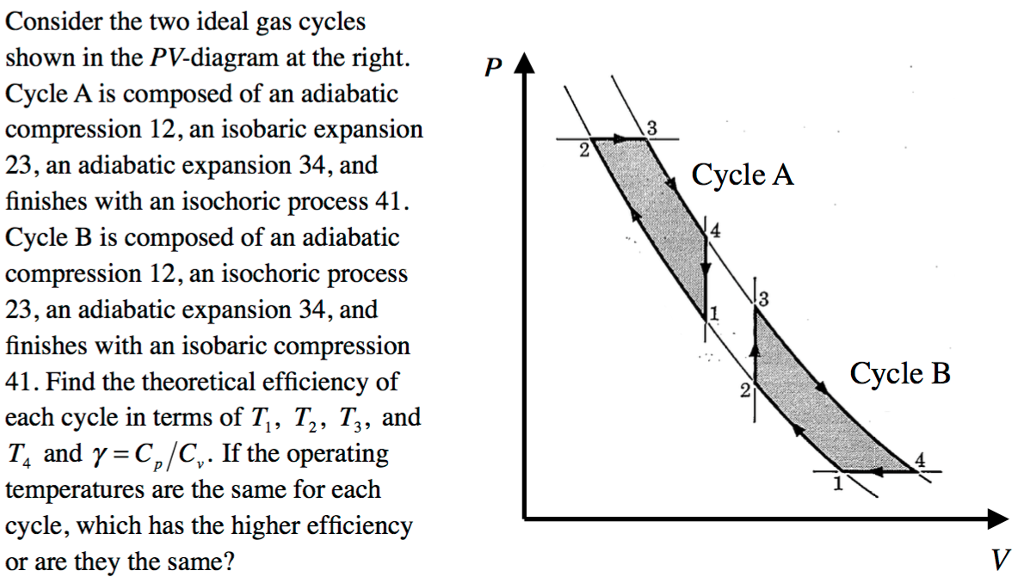
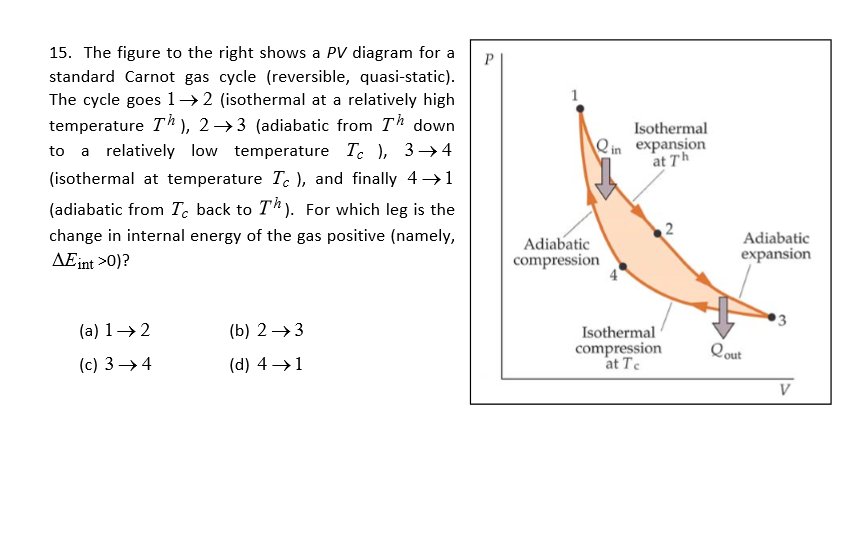
Adiabatic process - JEE First One mole of a monatomic ideal gas is taken along two cyclic processes and as shown in the PV diagram. The processes involved are purely isochoric, isobaric, isothermal or adiabatic. Match the paths in List I with the magnitudes of the work done in List II and select the correct answer using the codes given below the lists. Codes: Continue Reading
Difference between Isothermal and Adiabatic Process: Concept. Adiabatic process is a type of thermodynamic process in which where there is no heat transfer. In an adiabatic process, the system is insulated from the surroundings and no heat is absorbed or released. For an adiabatic process, the ideal gas formula will be, P V γ = c o n s t a n t γ is the ratio of specific heats at constant pressure and volume.
lambdageeks.com › adiabatic-compressionAdiabatic Compression: What Is, Working, Examples And ... The work done in an adiabatic process can be derived from the formula for adiabatic process. PV ꝩ = Constant (K). This formula can be rewritten as P=KV-ꝩ In order to calculate the work done in adiabatic process , let us consider the system is compressed from the initial position of P1, V1 and T1 to final position P2, V2 and T2. The work ...
mechanicalenotes.com › otto-cycleOtto Cycle: Process, PV Diagram, Efficiency with Derivation ... Process 1-2: Reversible Adiabatic Compression or Isentropic Compression: The air is compressed into the clearance volume of the cylinder and thus raising the pressure and temperature of the air. But, entropy remains constant as there is no heat transfer across the cylinder walls.
Power and Efficiency Equations for Compressor Calculations If n=γ=C P /C V, the compression is adiabatic compression (no heat exchange with the surrounding). Most gas compressions generally follow the adiabatic curve. Hence compressor equations are also based on adiabatic curve with n=γ, PV γ = constant. Let subscripts 1 and 2 stand for inlet and outlet process conditions of the compressor.
P-V plots for two gases during adiabatic process are shown in the ... Q: P-V plots for two gases during adiabatic process are shown in the figure. Plots 1 and 2 should correspond respectively to (a) He and O2 (b) O2 and He (c) He and Ar (d) O2 and N2 Click to See Ans…
In an isothermal process the internal energy? Explained by FAQ Blog What is ∆ U in adiabatic process? According to the definition of an adiabatic process, ΔU=wad. o C undergoes a reversible adiabatic expansion from 200. L to 800. ... from the ideal gas law PV = NkT, PV remains constant through an isothermal process. A curve in a P-V diagram generated by the equation PV = const is called an isotherm. For an ...
Adiabatic process - Wikipedia In thermodynamics, an adiabatic process (Greek: adiábatos, "impassable") ... We can solve for the temperature of the compressed gas in the engine cylinder as well, using the ideal gas law, PV = nRT (n is amount of gas in moles and R the gas constant for that gas). Our initial conditions being 100 kPa of pressure, 1 L volume, and 300 K of temperature, our experimental constant …
Is isentropic and adiabatic the same? - TimesMojo What is PV and TS diagram? Pressure-volume (P-V) and temperature-entropy (T-S) diagrams are often used as teaching. aids to describe refrigeration processes in introductory textbooks. They trace the path of a. hypothetical element of gas as it moves through a system during a complete thermodynamic. cycle.
PV Diagram Isobar - BrainMass First the gas expands isobarically until its volume doubles. Then the gas expands adiabatically. Then the gas is cooled isobarically and finally the gas is compressed adiabatically until it returns to its original state. (a) Draw a PV diagram. I am not certain how to draw an isobar or in what direction everything should run for this diagram.
During isothermal expansion the slope of pv graph? On a pV diagram, the process occurs along a line called isothermal curve or an isotherm. This curve has the equation p = constant / V. It can be derived from ideal gas law. In an ideal gas, molecules have no volume and do not interact. ... Why is the PV curve for adiabatic process? On a p-V diagram, the process occurs along a line ...
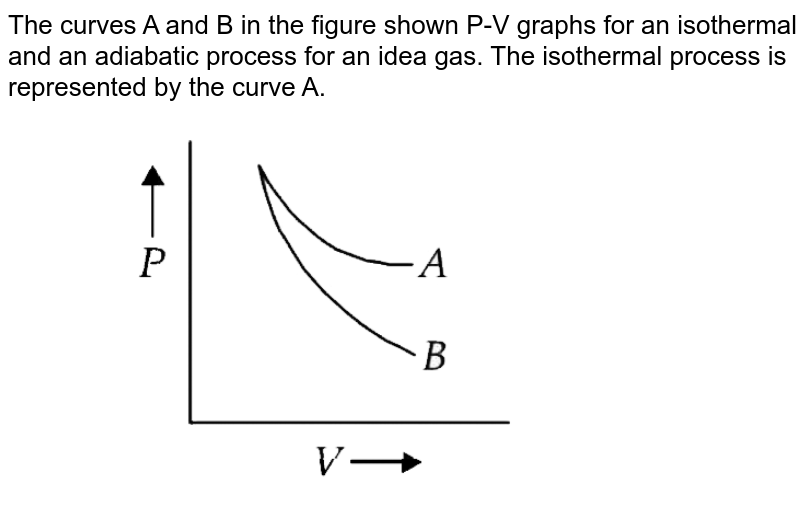
Cyclic processes on a PV diagram - JEE First Solution Along an isotherm remains constant whereas is constant for an adiabatic process. Since we identify the curve as an isothermal expansion and as an adiabatic one. The work done by the gas is simply the area under the PV curve. Since has the largest area under it, it must match the largest value on the second list, .
thermodynamics - How to justify that Carnot loop for ideal gas is ... Such adiabatic process can be done in many ways, including irreversible ones where the process can't be represented as a curve in PV diagram, for example, sudden expansion to decrease temperature, or sudden compression to increase it. But that would not be a reversible process.
Adiabatic Process - Definition, Types, Equation, Derivation, FAQs PV = RT [ for one mole of gas ] Substituting the value of P in the equation This equation gives the relation between V (Volume) and T (Temperature). Derivation Of Work Done In Adiabatic Process Go through the below derivation of work done in the Adiabatic process and
What is meant by isentropic? Explained by FAQ Blog An isentropic process is also known as a reversible adiabatic process. As this is an adiabatic process so no heat enters or leaves the system. On the other hand, Isobaric is a constant pressure and Isochoric is the constant volume process and Isothermal is the constant temperature process. ... Each point on a PV diagram corresponds to a ...
Calculator and Sketcher The new condition of the air is calculated and this new point and the process will be automatically drawn in the diagram. Customizations Set the chart according to your needs: Chart Style: Mollier/ Psychrometric-Chart. The x/y-axes will be flipped; Units-system can be set to Metric(SI) or Imperial(I-P).
Does isothermal mean adiabatic? Explained by FAQ Blog PV diagrams - part 2: Isothermal, isometric, adiabatic processes | MCAT | Khan Academy. 23 related questions found. ... The major difference between these two types of processes is that in the adiabatic process, there is no transfer of heat towards or from the liquid which is considered. Where on the other hand, in the isothermal process, there ...
Work Done in an Adiabatic Process - Physicscatalyst In and adiabatic process if W>0 i.e., work is done by the gas then T 2 < T 1 If work is done on the gas (W<0) then T 2 > T 1 i.e., temperature of gas rises. Solved Examples
Adiabatic process PV^y = Constant, y = Cp/Cv = 3/2: A short explanation The p-v diagram is drawn for the aforementioned described processes in the figure below. It can be seen in the image that the polytropic process is reduced to an isothermal process when n=1, the...
Isentropic process - Wikipedia In thermodynamics, an isentropic process is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in engineering as a model of and basis of comparison for real processes. This process is idealized because reversible …
When A Gas Undergoes An Isothermal Process, There Is In contrast an adiabatic process is where a system exchanges no heat with its surroundings (Q = 0). See also how much does a cameraman make What happens to the internal energy. Skip to content. Find Your Answer Menu. ... Work Heat & Internal Energy PV Diagrams; 12 What happens to the internal energy of a gas during adiabatic process?
Mollier-Diagram Calculator and Sketcher The new condition of the air is calculated and this new point and the process will be automatically drawn in the diagram. Customizations Set the chart according to your needs: Chart Style: Mollier/ Psychrometric-Chart. The x/y-axes will be flipped; Units-system can be set to Metric(SI) or Imperial(I-P). This affects the chart, input values and ...
P-V and T-S Diagrams - NASA 13.05.2021 · A process performed at constant temperature is called an isothermal process. During an adiabatic process no heat is transferred to the gas, but the temperature, pressure, and volume of the gas change as shown by the dashed line. As described on the work slide, the area under a process curve on a p-V diagram is equal to the work performed by a gas during the …
› what-is-adiabaticWhat is Adiabatic Expansion - Adiabatic Compression - Definition May 22, 2019 · An adiabatic process is a thermodynamic process, in which there is no heat transfer into or out of the system (Q = 0). The system can be considered to be perfectly insulated. In an adiabatic process, energy is transferred only as work. The assumption of no heat transfer is very important, since we can use the adiabatic approximation only in ...
Plotting actual PV diagram for air standard otto cycle and determine ... PV^gama=C- aplicable for both isentropic process. pv/t =c - aplicable to constant volume process . by using above 2 eqn we can find values of P and V at start and end of process (we will get ...
Adiabatic Compression: What Is, Working, Examples And … The work done in an adiabatic process can be derived from the formula for adiabatic process. PV ꝩ = Constant (K). This formula can be rewritten as P=KV-ꝩ In order to calculate the work done in adiabatic process , let us consider the system is compressed from the initial position of P1, V1 and T1 to final position P2, V2 and T2. The work ...
Thermodynamic: Laws of Thermodynamics, System Variables - Embibe Adiabatic Process. Adiabatic process is a thermodynamic process in which there is no exchange of heat between the system and the surrounding from the initial to the final state. Since the heat exchange is zero \(∆Q = \rm{const} = 0\) Internal energy of the system is given by, \(∆U = nC_v ∆T\) Equation of thermodynamic process is given by,
en.wikipedia.org › wiki › Isobaric_processIsobaric process - Wikipedia An isobaric process is shown on a P–V diagram as a straight horizontal line, connecting the initial and final thermostatic states. If the process moves towards the right, then it is an expansion. If the process moves towards the left, then it is a compression. Sign convention for work



















0 Response to "39 adiabatic process pv diagram"
Post a Comment