39 be2+ molecular orbital diagram
molecular orbital theory - Could Be2- exist? - Chemistry Stack Exchange But it seems to me that since the next electron would go into the π u orbital, which is a bonding orbital, Be 2- would have a bond order of 0.5, and Be 22- would have a bond order of 1, since it would have 4 in bonding (2 in σ and 2 in π)and only 2 in the σ*. Could Be 2- or Be 22- at least theoretically be produced? Draw the molecular orbital diagram of N2 and calculate the bond order ... The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Bond order formula is given as below. Bondorder=1/2 [a-b] where. a = Number of electrons in bonding molecular orbitals. b = Number of electrons in antibonding molecular orbitals. (i) Structure of N 2.
Be2 Molecular Orbital Diagram from the above mo diagram we can see that number of elctrons in the bonding and antibonding orbital is same and hence be does not form be2 molecule (for.may 04, · turning to [be2]^- we have [be2]^- 5 valence e⁻s: σ1 (2e⁻) σ2 (2e⁻) π1 (1e⁻) σ3 (0e⁻) π2* (0e⁻) σ4* (e⁻) bond order = ½ [σ (bonding e⁻) - σ (antibonding e⁻)] so if we take the bonding …

Be2+ molecular orbital diagram
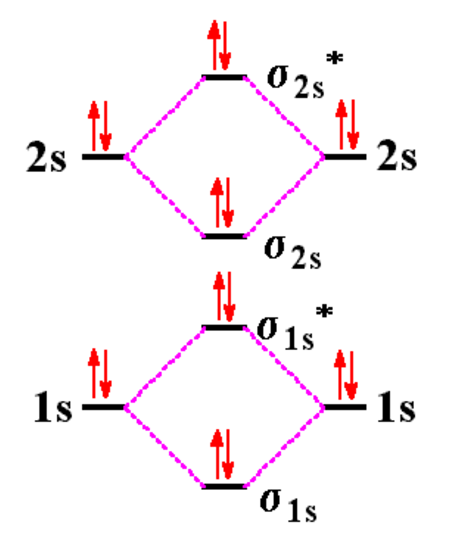
How to Make the Molecular Orbital Diagram for Be2+ (Bond Order ... How to Make the Molecular Orbital Diagram for Be2+ (Bond Order, Paramagnetic or Diamagnetic) 5,159 views Jan 17, 2021 This video discusses how to draw the molecular orbital (MO) diagram for the... Why does the molecular orbital diagram for Be2+ consist of 7 electrons ... For complete MO diagrams, you use the total number of electrons. The 1s electrons of O2, N2, etc. are used to fill up the sigma(1s) and sigma(1s)* molecular orbitals. Similarly, with Be2 + as well, there are 2(4) - 1 = 7 total electrons if you're filling out a complete MO diagram. Use molecular orbital theory to explain why Be2 molecule ... - Toppr Ask Solution. Be 2 molecule has MO electronic configuration KK(σs) 2(σ ∗2s) 2. The bond order is 22−2=0. Zero bond order indicates that Be 2 molecule does not exists.
Be2+ molecular orbital diagram. Molecular Orbital (MO) Diagram of Be2 - YouTube Molecular Orbital Diagram for Beryllium Dimer (Be2)Fill from the bottom up, with 4 electrons total.Bonding Order is 0, meaning it does not bond, and it is di... Solved 1. Draw the molecular orbital energy level diagram Draw the molecular orbital energy level diagram for each of the following species Be2+, Be2, and Be2-. Indicate theirnumbers of unpaired electron and mention ... What is the bond order for be2+? Hey guys, So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond. Is be2 2 paramagnetic or diamagnetic? Using the molecular orbital theory, why does a Be2 molecule not ... - Quora In combination with the final structure coordinates, you can draw a 3-D diagram with bonds drawn to link the atoms that the bonding table shows as having a bond. Structure coordinates (x,y,z for each atom) C 0.00000000 0.00000000 0.00000000 H -0.00000001 0.00000001 1.08363437 Continue Reading Bhimsara Chetry
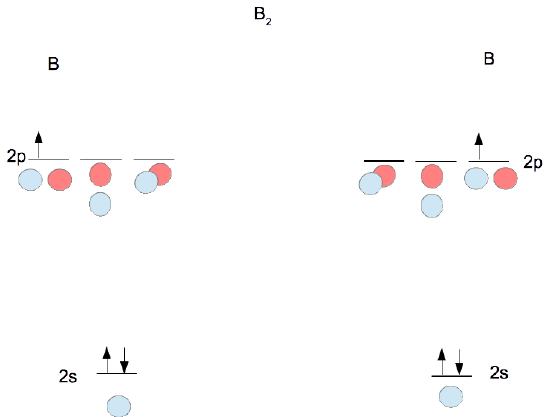
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... Draw MOT diagram for B2 molecule and calculate its class 11 ... - Vedantu The molecular orbitals having the same sign combine and give bonding molecular orbitals. We have to draw the molecular orbital diagram for B 2 molecule. The B 2 molecule is formed by the combination of two boron atoms. The two boron atoms are linked by a covalent bond. The atomic number of boron is 5. Draw the molecular orbital diagram for:(i) Be2(ii) B2 and predict bond ... Zigya App Draw the molecular orbital diagram for: (i) Be2 (ii) B2 and predict bond order and magnetic properties. 3056 Views Switch Flag Bookmark Explain the formation of H2 molecule on basis of valence bond theory. Or In the light of attractive and repulsive forces, show that a molecule of hydrogen is formed. 966 Views Answer Answered: Below are the molecular orbital… | bartleby What are the shapes of the molecular orbitals? (The diagram has been attached) Below are the molecular orbital diagrams for B2, C2, and N2. Please use it to predict the bond order for B2, B2+ ,B2 -, C2, C2+, C2 -, N2, N2+, and N2-. Which of these molecules or ions would be paramagnetic?
SOLVED:Draw an MO energy diagram and predict the bond order of Be2+ and ... If these core electrons than could combine into molecular orbital's, we would see a sigma one s molecular orbital. The bond order then because this is a bonding molecular orbital would be one half multiplied by these two electrons, giving us a bond order of one. Suggesting, yeah that B 2 2 Plus would be plausible. But let's back up a bit. SOLVED:Draw the molecular orbital diagram for BeC in this problem, we are asked to draw the molecular orbital diagram for the dia tom between beryllium and carbon. So ultimately what we're first going to do is separate it into two states beryllium and carbon and we'll write the electron configuration for each. So beryllium is one S 22 S two and carbon is one S 22 S two to P. Two. So for molecular orbital's would just draw the atomic orbital's ... Be2 Molecular Orbital Diagram - schematron.org Even rather simple molecular orbital (MO) theory can be used to predict which we start reading from the bottom of the diagram because this is how MO diagrams are constructed, Diberyllium, Be2, has a bond order of zero and is unknown. Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−. BeH2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity The sp hybrid orbital of the beryllium atom overlap with the 1s atomic orbital of the hydrogen atom, which is shown in the orbital diagram of the beryllium hydride molecule as follows: Hence, the VBT method also leads to the sp hybridization of the Beryllium atom in Beryllium hydride with linear geometry. BeH2 Polarity
Molecular Orbital Diagram Maker ©2022 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window
Energy level diagram for Molecular orbitals - Class Notes Bond order = 1. The molecule is paramagnetic. 9) C2. The electronic configuration of C2 is KK (σ (2s))2 (σ∗(2s))2 (π (2px))2 (π (2py))2. Nb= 6, Na =2. Bond order= 2. The molecule is diamagnetic. The double bond in C2 consist of both Pi bonds because the four electrons are present in the two pi molecular orbitals.
He2 Bond Order - what is an f2 bond order quora, solved determine the ... He2 Bond Order - 18 images - chem 101 week 11 ch10, molecular orbital theory could diatomic helium exist chemistry, why he2 cannot exist on the basis of mo theory peibdtmm chemistry, poem dilithium a bonus poem from the periodic table of poetry series,
Solved #15. The Molecular Orbital diagram below is | Chegg.com The Molecular Orbital diagram below is appropriate for Li2 and Be2. After completing the diagram for Li2 and Bez, determine which of the following statement is true. 02p* Izp* 62P IZP 023* 023 013* Ols A) both are stable and diamagnetic. B) Li2 is stable and diamagnetic, but Bez is unstable. C) Bez is stable and diamagnetic, but Liz is unstable.
Use molecular orbital theory to explain why the Be2 ... - SaralStudy The electronic configuration of Beryllium is 1s 2 2s 2. From the electronic configuration it is clear that there is no singly filled atomic orbital present in beryllium. Without the half- filled orbital, the overlapping is not possible, therefore Be2 molecule does not exist. Previous Question Next Question Popular Questions of Class 11 Chemistry
Solved Construct the molecular orbital diagram for Be2. Note - Chegg Expert Answer. Transcribed image text: Construct the molecular orbital diagram for Be2. Note that the 1s orbitals are not shown. Be Ho Be Answer Bank IL | Identify the bond order. O 0 O os O 1s.
Molecular Orbital Diagram Be2 Draw the molecular orbital energy level diagram for each of the following species Be2+, Be2, and Be Indicate theirnumbers of unpaired electron and mention. The first ten molecular orbitals may be arranged in order of energy as follow: σ (1s ) ∗ (1s) Molecular orbital energy level for Be2.
7.7 Molecular Orbital Theory - Chemistry Fundamentals molecular orbital diagram ( Figure 7.7.9 ). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The molecular orbitals formed by the combination of the atomic orbitals are shown in the center.
o2 molecular orbital diagram Diagram mo bn molecular orbital theory ... If you are looking for Draw the molecular orbital energy level diagram of H2 molecule - Brainly.in you've visit to the right page. We have 9 Pictures about Draw the molecular orbital energy level diagram of H2 molecule - Brainly.in like I'm pretty sure the diagram for O2 and F2 is wrong - 1574215, why oxygen is paramagnetic in nature by molecular orbital theory and also I'm pretty sure ...
What is the molecular orbital diagram for B_2? | Socratic Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals.
BeCl2 Lewis Structure, Molecular Geometry ... - Techiescientist The molecular orbital diagram of the BeCl2 molecule is drawn by the combination of Beryllium atomic orbitals and chlorine group orbitals. As there are two chlorine atoms and hence, first they combine to form group orbitals. The electronic configuration of Cl is [Ne] 3s23p5.
The molecular electronic configuration of Be2 is: - Toppr Ask >> Molecular Orbital Theory >> The molecular electronic configuration o Question The molecular electronic configuration of Be 2 is: A σ1s 2,σ ∗1s 2,σ2s 2,σ ∗2p 2 B σ1s 2,σ2s 2 C σ1s 2,σ ∗1s 2,σ2s 2,σ ∗2s 2 D none of the above Medium Solution Verified by Toppr Correct option is C) Total no. of electrons in Be 2 =8.
Use molecular orbital theory to explain why Be2 molecule ... - Toppr Ask Solution. Be 2 molecule has MO electronic configuration KK(σs) 2(σ ∗2s) 2. The bond order is 22−2=0. Zero bond order indicates that Be 2 molecule does not exists.
Why does the molecular orbital diagram for Be2+ consist of 7 electrons ... For complete MO diagrams, you use the total number of electrons. The 1s electrons of O2, N2, etc. are used to fill up the sigma(1s) and sigma(1s)* molecular orbitals. Similarly, with Be2 + as well, there are 2(4) - 1 = 7 total electrons if you're filling out a complete MO diagram.
How to Make the Molecular Orbital Diagram for Be2+ (Bond Order ... How to Make the Molecular Orbital Diagram for Be2+ (Bond Order, Paramagnetic or Diamagnetic) 5,159 views Jan 17, 2021 This video discusses how to draw the molecular orbital (MO) diagram for the...









0 Response to "39 be2+ molecular orbital diagram"
Post a Comment