42 lewis dot diagram for phosphorus
draw the lewis structure for the phosphorus trichloride(PCL3) molecule ... How many electrons should be included in the Lewis Dot Structure for phosphorus trichloride (PCl3)? 1.A 12.39g sample of phosphorus reacts with 45.4 g of Chlorine to form Phosphorus Trichloride(PCl3). if... PCl3 Lewis Structure - How to Draw the Lewis Structure for PCl3 ... A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure (Phosphorus Trichloride).For the PCl3 structure use the periodic table to find the tot...
PI3 Lewis Structure: How to Draw the Lewis Structure for PI3 ... PI3 is also called Phosphorus triiodide. ----- Steps to Write Lewis Structure for compounds like PI3 ----- 1. Find the total valence electrons for the PI3 molecule. 2. Put the least electronegative...

Lewis dot diagram for phosphorus
How to draw PF3 Lewis Structure? - Science Education and Tutorials In the PF3 Lewis structure diagram, the phosphorus atom can be the center atom of the molecule. As a result, central phosphorus in the PF3 Lewis structure, with all three fluorine atoms arranged in a trigonal pyramidal geometry. Add valence electrons around the fluorine atom, as given in the figure. Lewis Electron Dot Diagrams | Introductory Chemistry - 1st Canadian Edition What is the Lewis electron dot diagram for each element? phosphorus argon Answer For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4 s2 3 d6) is as follows: How to Draw the Lewis Dot Diagram of P (Phosphorus) - YouTube With elemental phosphorus (white phosphorus, P4) as a bonus.Check me out:
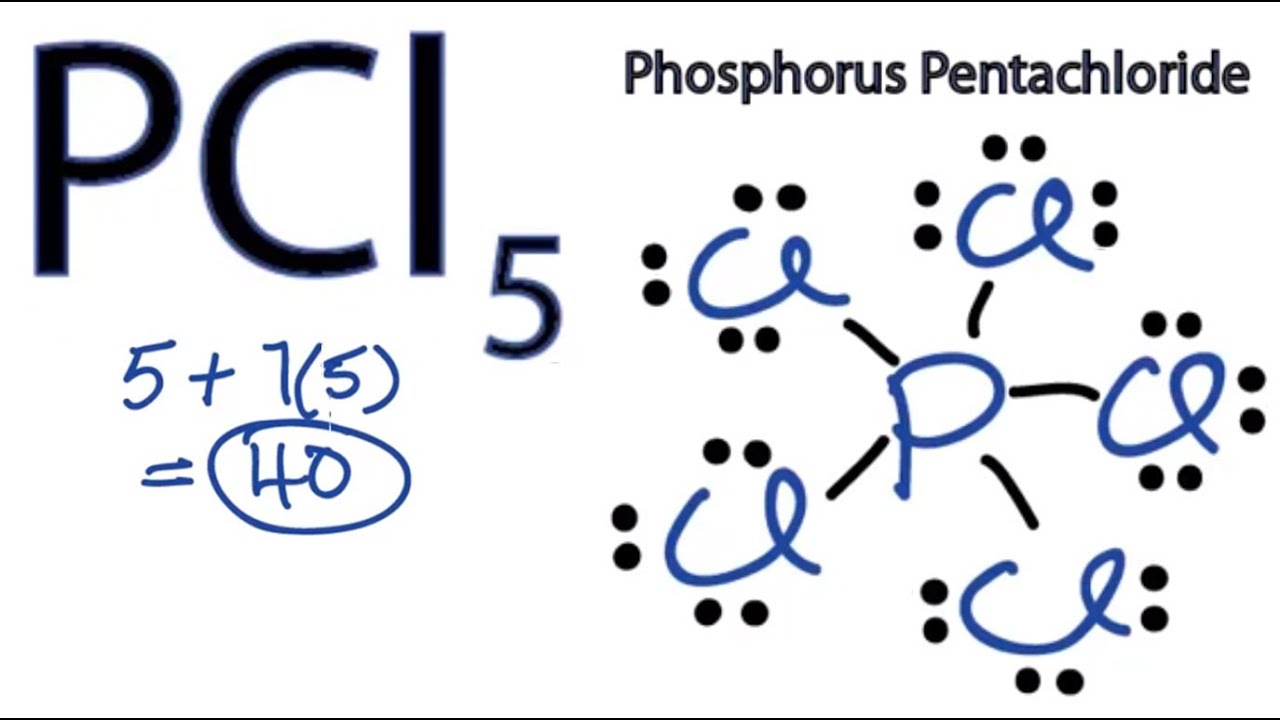
Lewis dot diagram for phosphorus. PCl3 (Phosphorus Trichloride) Lewis Structure PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories. Lewis Electron Dot Diagrams - Introductory Chemistry, 1st Canadian ... What is the Lewis electron dot diagram for each element? phosphorus argon Answer For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4 s2 3 d6) is as follows: P2O5 (Phosphorus pentoxide) Lewis Structure Steps of the drawing lewis structure of P 2 O 5 molecule are explained in detail in this tutorial. P 2 O 5 lewis structure In this lewis structure, center oxygen atom has made single bonds with two phosphorus atoms. Each phosphorus atom has made bonds with three oxygen atoms. There are four (P=O) double bonds in this molecule. Lewis Structure of PCl5 (With 5 Simple Steps to Draw!) - Knords Learning The Phosphorus atom (P) is at the center and it is surrounded by 5 Chlorine atoms (Cl). The Phosphorus atom does not have a lone pair while all the 5 Chlorine atoms have 3 lone pairs. Let's draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am ...
Phosphorus Lewis Dot Structure: Drawing, Several Compounds And Detailed ... Phosphorus ion lewis dot structure The Phosphorus ion that we are going to study in this section is Phosphate ion (PO43-). The valence electrons in phosphorus are 3 and in oxygen, there are 6 valence electrons (since 4 oxygen atoms are present the total valence electrons will be 4×6=24) and also 2 more electrons due to the -2 charge on the ion. Lewis Electron Dot Diagrams - Introductory Chemistry - 1st Canadian Edition A. Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... Lewis Dot Structure for Phosphorous Atom (P) - YouTube After that I draw the Lewis dot structure for Phosphorous (P). Note: Phosphorous is in Group 15 (sometimes called Group V or 5). Since it is in Group 5 it will have 5 valence electrons. When you... PBr3 Lewis Structure, Molecular Geometry, Hybridization and Polarity There are 26 valence electrons for Phosphorus Tribromide. PBr3 Lewis Structure Lewis dot structures or Lewis structures are the diagrams that help to understand the bonding of atoms along with the lone pairs present in the molecule. The valence electrons of atoms form bonds, and these bonds are represented by showing straight lines.
How would you draw a Lewis structure for an atom that has the electron ... A P with 5 dots surrounding it. Based on your electron configuration, the element that you want to draw is Phosphorus, since you have 15 electrons. Phosphorus has 5 valence electrons. To draw Lewis Structures for elements, the symbol for the element is drawn with the number of valence electrons it has surrounding it. So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus ... Phosphorus trifluoride (PF3) lewis dot structure, molecular geometry ... To calculate the formal charge in the PF3 lewis structure. Use the formula given below- Let's start with the central atom which is phosphorous in the PF3 molecule. For phosphorous atom: ⇒ Valence electrons of phosphorous = 5 ⇒ Lone pair electrons on phosphorous = 2 ⇒ Bonding electrons of phosphorous (3 single bonds) = 6 Iodine Lewis Dot Structure: Drawing, Several Compounds and Detailed ... Lewis dot structure for phosphorus iodine: Structure of Phosphorus triiodide (PI 3): Iodine contains seven valence electrons, whereas phosphorus has five, for a total of 26. Phosphorus is the least electronegative element.The core element, phosphorus, makes connections with three iodine atoms, and thus Phosphorus and Iodine complete their octet. What is the Lewis Dot Structure for Phosphorus trichloride? There are 3xx7+5=26 valence electrons to distribute, i.e. 13 " electron pairs". Around EACH bound Cl atom there are 3 lone pairs; there are 3xxP-Cl bonds; the thirteenth lone pair resides on phosphorus: :P(-Cl)_3. Since there are 4 electron pairs around phosphorus, the geometry is based upon a tetrahedron, but since one of these electron pairs is a stereochemically active non-bonding pair, the ...
How Many Dots Are Drawn In The Lewis Dot Diagram For Phosphorus So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus, the letter P. Next, Phosphorus has 5 valence electrons. So start with one dot on top, then one dot to the right, one dot on the bottom, one dot to the left, and another dot on top, next to the first one. How many Lewis dots are in phosphorus?
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry - Lumen Learning A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Solved Phosphorus Draw the Lewis dot structure for | Chegg.com Question: Phosphorus Draw the Lewis dot structure for phosphorus. Include all lone pairs of electrons Include all lone pairs of electrons This problem has been solved!
10.1 Lewis Electron Dot Diagrams - Introductory Chemistry - NSCC Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
PO43- Lewis Structure - Learnool PO 43- (phosphate) has one phosphorus atom and four oxygen atoms. In the lewis structure of PO 43-, there is one double bond and three single bonds around the phosphorus atom, with four oxygen atoms attached to it. One oxygen atom with a double bond has two lone pairs, and three oxygen atoms with single bonds have three lone pairs.
PF5 Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair And ... So in the below sections, we will understand the phosphorus pentachloride lewis structure and other facts about it in detail. Some facts pentafluorophosphorane PF5 has a molecular weight equal to 125.96 g/mol. In appearance, it is observed to be a gas which is colorless and has one so pleasant odor. It's observed density is around 5.527 kg/m3.
Lewis dot diagram for phosphorus? - Answers Lewis dot diagram for phosphorus. Wiki User. ∙ 2008-03-05 01:49:07. Study now. See answer (1) Best Answer. Copy.
How to Draw the Lewis Dot Structure for Sr3P2: Strontium phosphide A step-by-step explanation of how to draw the Sr3P2 Lewis Dot Structure.For Sr3P2 we have an ionic compound and we need to take that into account when we dra...

0 Response to "42 lewis dot diagram for phosphorus"
Post a Comment