38 orbital diagram of copper
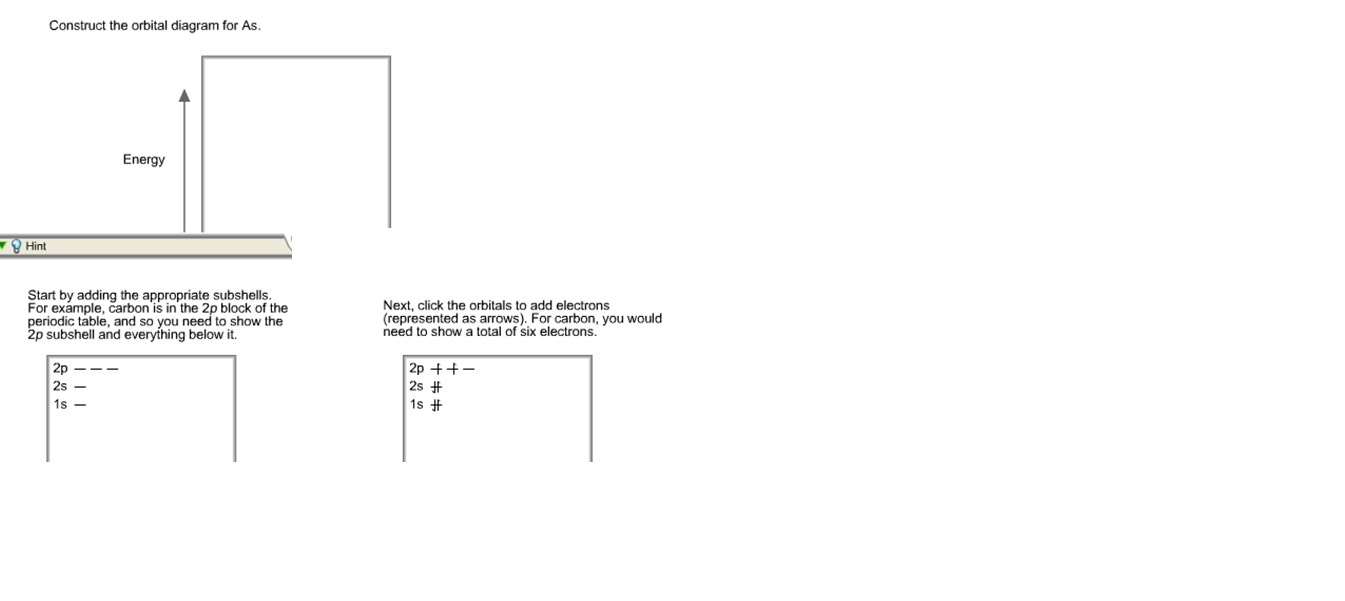
4) Write the orbital filling (arrow) diagram of the valence electrons ... Write the following for an atom of Copper: (3pts) a. Condense orbital diagram Group #:_ b. Full electron configuration C. How many valence electrons does a cheomitam atom have? Copper nen an electron moves from n=4 to n=2 in the hydrogen atom, energy is released in form of a photon (light particle). Calculate the wavelength of this photon in ... Diagram Orbital Copper - mds.fabbro.fvg.it an element's electron configuration can be represented using energy level diagrams, or aufbau diagrams in reality, copper has full 3d orbital and a half-full 4s orbital, which is a more stable configuration so, for example, if we wanted to know the electron configuration for sodium (atomic number 11), we start at the top left and follow that …
Copper Bohr Model - Learnool For a detailed explanation, check the orbital diagram of copper. #6 Draw 4 th Electron Shell. The 4 th electron shell (containing s subshell, p subshell, d subshell, and f subshell) can hold up to a maximum of 32 electrons. So draw the 4 th electron shell as follows:
Orbital diagram of copper
How to Write the Atomic Orbital Diagram for Copper (Cu) To write the orbital diagram for the Copper (Cu) first we need to write the electron configuration for just . To do that we need to find the number of elect... Electron configuration - Wikipedia Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons … Electrochemical CO2 reduction (CO2RR) to multi-carbon products … 1.3.2022 · It is generally accepted that CO 2 reduction over metallic copper and copper-based materials is favorable for the formation of several hydrocarbons and oxygenate products , .The reduction process is governed by a multi-step-based coordination chemistry comprising two, six, eight, and twelve electrons for the formation of the common products CO, CH 3 OH, CH 4, C 2 H …
Orbital diagram of copper. WebElements Periodic Table » Copper » properties of free atoms Copper: properties of free atoms. Cu. Available copper properties... More properties... Copper atoms have 29 electrons and the shell structure is 2.8.18.1. The ground state electron configuration of ground state gaseous neutral copper is [ Ar ]. 3d10. 4s1 and the term symbol is 2S1/2. Orbital Diagram Copper [F8DVUI] - discover.vda.it What is Copper Orbital Diagram. Likes: 617. Shares: 309. Electron configuration for Copper (element 29). Orbital diagram Cu (Copper) is an element with position number 29 in the periodic table. Located in the IV period. Melting point: 1083.5 ℃. Density: 8.92 g/cm 3 . The order of filling the orbitals with electrons in the Cu atom is an exception to the rule. Expected electronic configuration. 1s2 2s2 2p6 3s2 3p6 4s2 3d9. But in reality, one electron moves from ... SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram … 1 day ago · The molecular orbital diagram of SO2 is attached below: A molecular orbital diagram gives us an idea about how the atomic orbitals of two different atoms can fuse and give rise to a new orbital. This further helps us to find out the bond order, bond length, and bond strength of any compound.
Molecular orbital diagram for two coordination modes of... | Download ... Using [(mhd)Cu (BTMSA)] for copper CVD, adhesive, continuous and pure copper metallic thin films (< 1 % impurity as seen by XPS) were grown on Ta/TaN/ SiO2/Si substrates with an optimized growth ... Schematic molecular orbital diagram for Cu( II / I ) complexes showing ... The major delocalisation of electron density in the copper d yz orbital occurs in the sp 2 hybridised orbitals on the nitrogen donors and the in- plane p orbitals on the sulfur atoms. These spin... SO2 Molecular Geometry, Hybridization, Lewis Structure & MO Diagram This adds up to the explanation of the molecular orbital diagram of SO2. Similarities between Sulfur and Oxygen atoms. Both O and S have the same outer electric configuration of ns2 and np4. O and S are usually divalent. O and S are non-metals. Both exhibit allopatric form. In reaction with metals, both react with the oxidation state of -2. Chemistry Chapter 7 Flashcards | Quizlet B) f orbital Basically, when l = 0 the is one orbital and it is called an s-orbital. It can hold 2 electrons total. When l = 1, the orbitals are called p-orbitals and there are 3 of them. Each of the individual p-orbitals can hold 2 electrons each. This gives us a total of 6 electrons that can go into the 3 p-orbitals.
Diagram Copper Orbital anomalous electron configuration example: chromium copper 14 in an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally in an atom there are levels of energy in the skin and sub skin copper (cu) has an atomic mass of 29 write the abbreviated … Copper(Cu) electron configuration and orbital diagram - Valenceelectrons To write the orbital diagram of copper (Cu), you have to do the electron configuration of copper. Which has been discussed in detail above. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. ... Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital ... Orbital Diagram Copper - gokudzuke.uglchimici.sardegna.it Elements Orbital Diagram Electron Configuration Be 4 ↑↓ ↑↓ 1s 2s 1s 2 2s 2 1 the orbital diagrams, for the following elements (D) Copper atoms contain more electrons than silicon atoms Pauli Exclusion Principle: orbitals may contain a maximum of two electrons, and these must have opposite The next step is to start drawing an orbital ...
Orbital Diagram For Calcium (Ca) | Calcium Electron Configuration The 6 electrons will go to the 2p orbital, and the next 2 electrons will place with the 3s orbital, and now we have only 8 electrons in which 6 electrons will go with the 3p orbital, and the last 2 electrons will be with the 4s orbital. So, we have Calcium Electron Configuration is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s². So, the configuration helps ...
40 Electron Configurations, Orbital Box Notation (M7Q7) - Unizin The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +½).
Chemistry - Learnool The orbital diagram of copper shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p subshell has 6 electrons, the 4s subshell has 1 electron, and the 3d subshell has 10 electrons. Steps Here's how you can … Read more Nickel Orbital Diagram
Cobalt(Co) electron configuration and orbital diagram To create an orbital diagram of an atom, you first need to know Hund’s principle and Pauli’s exclusion principle. Hund’s principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way.
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Uparrow goes first then, downarrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Chem4Kids.com: Copper: Orbital and Bonding Info It is located in the fourth row (period) on the table of elements and is one of three elements that has one electron in its outer orbital. Potassium and chromium are the other two. Its electron configuration of 2-8-19-1 allows it to work well as a pure element and in a variety of compounds. More about the history and places to find copper.

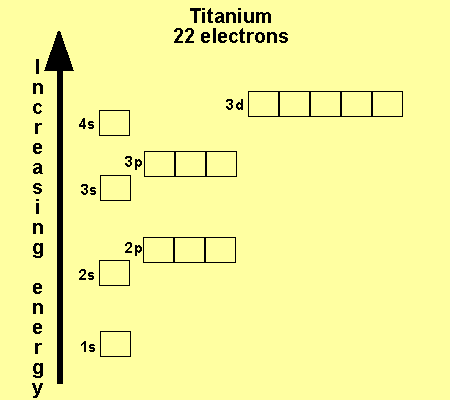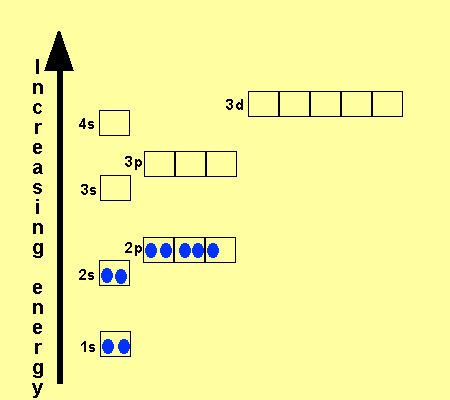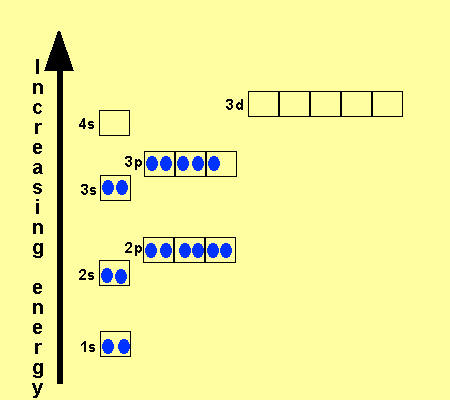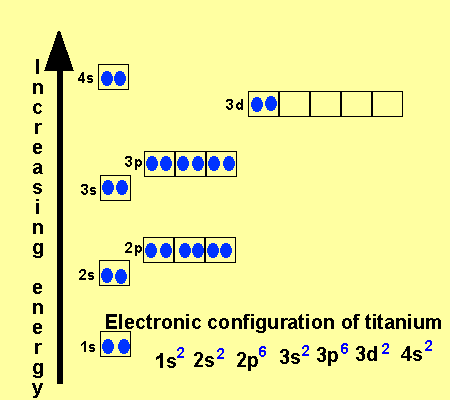
0 Response to "38 orbital diagram of copper"
Post a Comment