38 molecular orbital diagram for ne2 2+
Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. Molecular Orbital MO Diagram for N2(2-) - YouTube the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ...
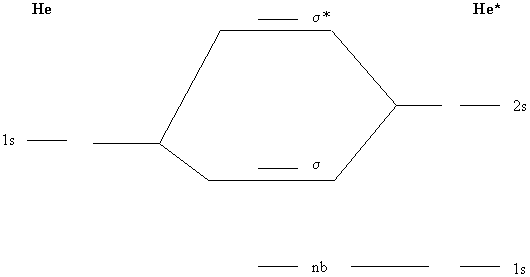
Molecular Orbital Diagram For He2 The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = . The molecular orbital energy-level ...

Molecular orbital diagram for ne2 2+
What are the molecular orbital configurations for N_2^+, N_2 ^(2+), N_2 ... If we build the MO diagram for "N"_2, it looks like this: First though, notice that the p orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be. Anyways, for the electron configurations, you would use a notation like the above. g means "gerade", or even symmetry upon inversion, and u means "ungerade", or odd symmetry upon inversion. It's not ... SOLVED:Draw out the molecular orbital diagram for Ne2, starting with ... Draw out the molecular orbital diagram for Ne2, starting with the 2s atomic orbitals and label each molecular orbital with the appropriate notation as done in class, i.e., σ*2s or Π2p, etc. Calculate the bond order of Ne2 and determine if you would expect this molecule to exist. Then write out the electron configuration for the molecule, and ... 7.7 Molecular Orbital Theory - Chemistry Fundamentals A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the [latex]\sigma[/latex] 1s bonding orbital; the electron configuration is [latex]{\left({\sigma}_{1s}\right)}^{2}.[/latex] We represent this configuration by a molecular orbital energy diagram (Figure 7.7.10) in which a single upward arrow indicates one ...
Molecular orbital diagram for ne2 2+. Solved Draw out the molecular orbital diagram for Ne2, | Chegg.com Draw out the molecular orbital diagram for Ne2, starting with the 2s atomic orbitals and label each molecular orbital with the appropriate notation as done in class, i.e., σ*2s or Π2p, etc. Calculate the bond order of Ne2 and determine if you would expect this molecule to exist. Then write out the electron configuration for the molecule, and ... Draw the molecular orbital diagram of N2 and calculate the bond order ... Bond order formula is given as below. Bondorder=1/2 [a-b] where. a = Number of electrons in bonding molecular orbitals. b = Number of electrons in antibonding molecular orbitals. (i) Structure of N 2. Electronic configuration of N 2 (14 electrons) = (σ1s) 2 (σ*1s) 2 (σ2s) 2 (σ*2s) 2 (π) 4 (2p z) 2. a = 10. Draw the molecular orbital diagram of N2N2 - Vedantu Molecular orbital diagram of N 2 + is shown below: This picture shows the molecular orbital diagram of N 2 +. Orbitals represented by ∗ are antibonding orbitals and the orbitals without ∗ are bonding orbitals. Bond order can be calculated by the formula: Bond order = bonding electrons - antibonding electrons 2. Draw a molecular orbital diagram of ${N_2}$ or ${O_2}$ with ... - Vedantu Lets calculate the bond order for ${N_2}$ : The total number of electrons present in the ${N_2}$ molecule is 14. Number of electrons in bonding orbitals : 8 Number of electrons in antibonding orbitals : 2 So, the formula to find bond order is Bond order = $\dfrac{1}{2}$ (Number of electrons in BMO) - (Number of electrons in ABMO)
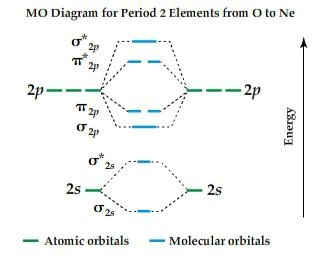
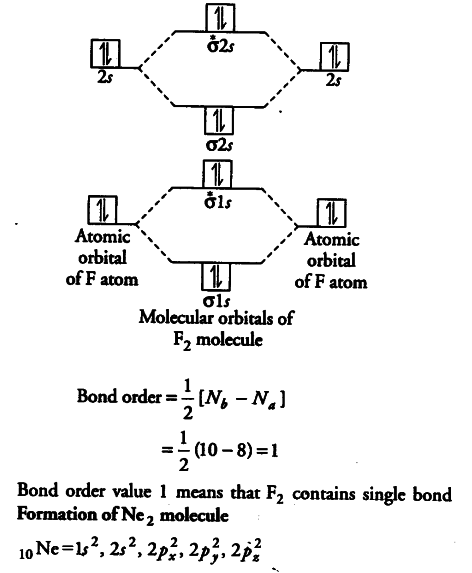
Molecular orbital (MO) diagram for N2 and N2^- - Chemistry Stack Exchange I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For N X 2 the orbitals in increasing energy are: σ 1 s < σ 1 s ∗ < σ 2 s < σ 2 s ∗ < π 2 p x, π 2 p y < σ 2 p z < π 2 p x ∗, π 2 p y ∗ < σ 2 p z ∗. because it has 14 electrons. Why does the Ne2 molecule not exist using molecular orbital theory? Answer (1 of 4): Molecular orbital confuguration of Ne2 is σ1s²σ*1s²σ2s²σ*2s²σ2Pz²π2Px²π2Py²π*2Px²π*2Py²σ*2Pz² Hence the bond order of Ne2 according to M.O.T is =(Nb-Na)/2 =(10-10)/2 (since,both bonding orbitals and non-bonding orbital contains 10 electrons) =0 Hence, no bond is possible b... Solved Draw the molecular orbital diagram for Ne2+ and | Chegg.com Draw the molecular orbital diagram for Ne2+ and determine if the bond between the two atoms will be stable. If 2p orbitals on an atom are all the same energy, why do they form molecular orbitals of different engergies when theu mix? Question: Draw the molecular orbital diagram for Ne2+ and determine if the bond between the two atoms will be ... rolls royce phantom user wiring diagram [DIAGRAM] Valence Molecular Orbital Diagram For Nitrogen Vs Oxygen FULL officephonesla.com.amandine-brevelay.fr. orbital ne2 nitrogen valence. Rolls Royce Silver Cloud 3 Factory Workshop Manual & Parts List | EBay . royce. Rolls royce silver cloud 3 factory workshop manual & parts list.
Molecular Orbital Theory | Energy Level Diagrams for O2, O2+, O2-, O2 2 ... In this video lecture, the MOEL for different Oxygen species lke O2, O2+, O2-, O2 2- , F2 and Ne2 are elaborated. Their electronic configurations, bond order... Molecular Orbital Diagram of O2, F2, and Ne2 Molecules. - YouTube 0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d... Molecular Orbital Diagram For Ne2 According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or. Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. © Prof Adam J Bridgeman | close window. © Prof Adam J Bridgeman | close window.The orbital correlation diagram in predicts the same thing--two electrons fill a single bonding molecular orbital. OneClass: ne2+ molecular orbital diagram Get the detailed answer: ne2+ molecular orbital diagram OneClass: ne2+ molecular orbital diagram 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION →
Molecular Orbital (MO) Diagram of N2 - YouTube Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
Molecular orbital diagrams of Cl2, H2O, and Br2. - ResearchGate Download scientific diagram | Molecular orbital diagrams of Cl2, H2O, and Br2. from publication: Theoretical Study of the Potential Energy Surfaces of the Van Der Waals H 2 O−X 2 + (X = Cl or Br ...
molecular orbital of ne2 - Brainly.in Brainly User. Molecular orbital diagram of. •Neon atom has 10 electrons and its electronic configuration is considered, it has two neon atoms and thus is composed of. The electronic configuration and bond order ofElectronic configuration: Bond order =. =. As the bond order value forN_2, it is unstable and cannot exist.
MO Diagram for N2+ (Molecular Orbital) - YouTube There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
Li2 Mo Diagram In this section, we will compare MO diagrams for diatomic molecules X-X, from Li 2 to Ne2. We will predict their bond order and see how the. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear ...
Use the molecular orbital energy level diagram to show that N2 would be ... Click here👆to get an answer to your question ️ Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2 , a single bond and Ne2 , no bond.
Is NE2 2+ paramagnetic or diamagnetic? - Quora Within that document is this diagram: As you can see, Ne2 has all of its orbitals (both bonding and antibonding—labeled with a *) filled. To form the 2+ ion, the uppermost electrons in the sigma* 2p orbital are removed, making it isoelectronic with F2, so it has a bond order of 1 and should be observable, though highly reactive.
(Get Answer) - Use the molecular orbital diagram below to determine the ... Use the molecular orbital diagram below to determine the bond order for (1) Ne2 and (2) 02 `1) The bond order of Ne2 is 2) The bond order of O2 is 2p ... Molecular Orbital Diagram The molecular orbital diagram below may be used for the following problem(s). However, the diagram will still yield the corect bond oeder and magnetic behavior for ...
write the molecular orbital diagram of n2 and calculate their bond ... A. give method and find hybridisation and shape of : 1. XeF2 2.SF2 3. 4.IF7 5. Molecular orbital theory explanation How many nodal planes do the SIGMA Pz ABMO.have and How? why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric.
7.7 Molecular Orbital Theory - Chemistry Fundamentals A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the [latex]\sigma[/latex] 1s bonding orbital; the electron configuration is [latex]{\left({\sigma}_{1s}\right)}^{2}.[/latex] We represent this configuration by a molecular orbital energy diagram (Figure 7.7.10) in which a single upward arrow indicates one ...
SOLVED:Draw out the molecular orbital diagram for Ne2, starting with ... Draw out the molecular orbital diagram for Ne2, starting with the 2s atomic orbitals and label each molecular orbital with the appropriate notation as done in class, i.e., σ*2s or Π2p, etc. Calculate the bond order of Ne2 and determine if you would expect this molecule to exist. Then write out the electron configuration for the molecule, and ...
What are the molecular orbital configurations for N_2^+, N_2 ^(2+), N_2 ... If we build the MO diagram for "N"_2, it looks like this: First though, notice that the p orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be. Anyways, for the electron configurations, you would use a notation like the above. g means "gerade", or even symmetry upon inversion, and u means "ungerade", or odd symmetry upon inversion. It's not ...





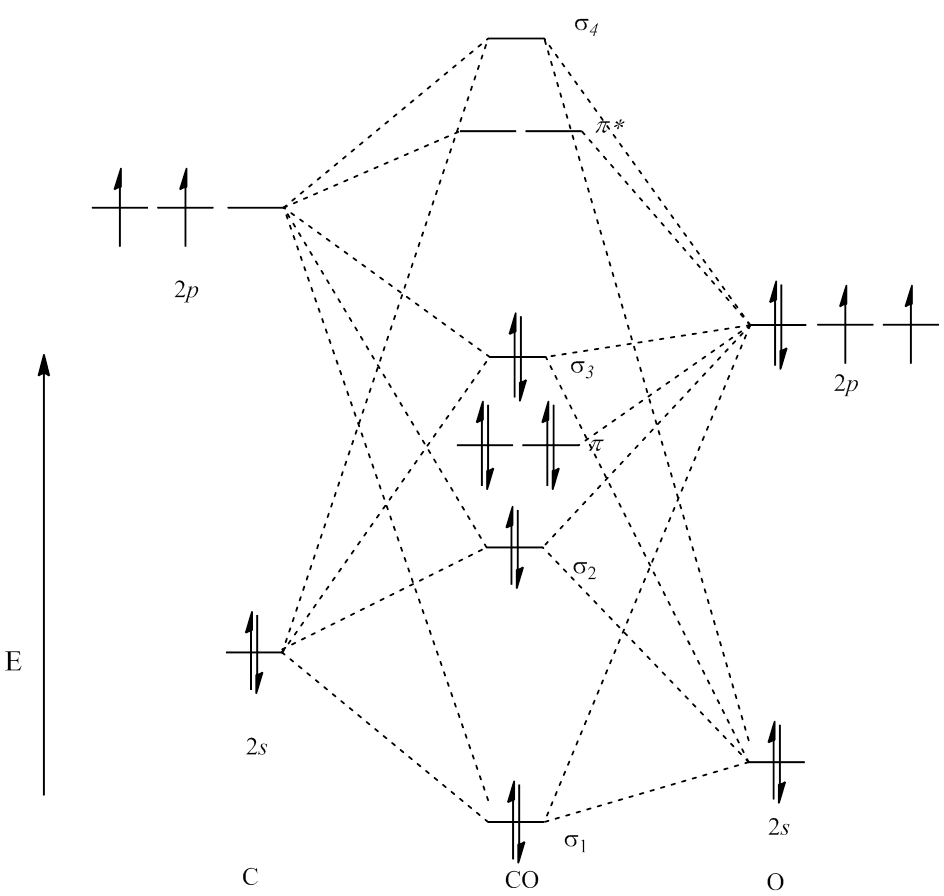


0 Response to "38 molecular orbital diagram for ne2 2+"
Post a Comment