38 atomic orbital diagram nitrogen
Electron Configuration for Nitrogen (N) - UMD In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration will be 1s 2 2s 2 2p 3. How to draw Bohr diagram for Nitrogen(N) atom - Topblogtenz Here, we will draw the Bohr diagram of the Nitrogen atom with some simple steps. Steps to draw the Bohr Model of Nitrogen atom 1. Find the number of protons, electrons, and neutrons in the Nitrogen atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).

Atomic orbital diagram nitrogen
Nitrogen | N2 - PubChem Nitrogen | N2 | CID 947 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. National Institutes of Health. National Library of Medicine. National Center for Biotechnology Information ... Nitrogen Orbital diagram, Electron configuration, and ... - Topblogtenz The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and the rest three electrons in the 2p orbital. The orbital diagram for a ground-state electron configuration of a nitrogen atom is as follows- Nitrogen Electron Configuration (N) with Orbital Diagram Jan 21, 2021 — The electron configuration of nitrogen is 1s2 2s2 2p3. You can see the image below. What is the Electron Configuration of Nitrogen. What is the ...
Atomic orbital diagram nitrogen. Show the orbital-filling diagram for N (nitrogen). - ZuoTi.Pro help Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Orbital-filling diagram Nitrogen - Periodic Table Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.The chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass. a) Molecular orbital diagrams of dinitrogen formed by combination of ... a) Molecular orbital diagrams of dinitrogen formed by combination of nitrogen atomic orbitals. b) Simplified schematic illustration of end‐on and side‐on bonding models, and the first activation... Nitrogen - Element information, properties and uses | Periodic Table The atomic number of each element increases by one, reading from left to right. Block Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Atomic number
What is the atomic orbital diagram for nitrogen? - Study.com What is the atomic orbital diagram for nitrogen? Atomic Orbital Diagrams: These are also known as electron-in-a-box diagrams. This is a simplified diagram of how the electrons are arranged within the orbitals for a particular atomic species. Answer and Explanation: 1. Nitrogen. Solved Fill in the atomic orbital diagram for nitrogen. - Chegg Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (18 ratings) Transcribed image text: Fill in the atomic orbital diagram for nitrogen. Answer Bank Energy Construct the orbital diagram for nickel. 1000 Answer Bank Energy 2 _ _ _. Nitrogen - Wikipedia Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System.At standard temperature and pressure, two atoms of the element bind to form N 2, a colorless ... Nitrogen - Atomic Number - N - Periodic Table Atomic Number of Nitrogen Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... What is the orbital diagram for a ground-state nitrogen atom ... - Answers A nitrogen atom has 3 orbitals; the 1s orbital, the 2s orbital, and the 2p orbital. In this case, the 2s and 2p orbitals are the valence orbitals, as they have the electrons with the most energy ... Nitrogen energy-level diagram - Big Chemical Encyclopedia A typical energy level diagram of inner n and n molecular orbitals of the bent azo group, N—N, in the ground state is shown in Fig. 2,a (41). Here, a 7t molecular orbital containing two electrons, as well as a vacant n orbital, result from the interaction of pz nitrogen atomic orbitals not involved in the sp2 hybridization of the N atoms. Atom Diagrams: Electron Configurations of the Elements - ThoughtCo For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.
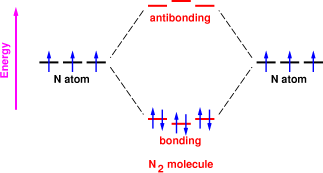
Molecular orbitals in Nitrogen - ChemTube3D There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two perpendicular pi bonds. Three filled bonding orbitals… … and three empty antibonding orbitals.
How to Write the Orbital Diagram for Nitrogen (N) - YouTube To write the orbital diagram for the Nitrogen atom (N) first we need to write the electron configuration for just N. To do that we need to find the number o...
Nitrogen(N) electron configuration and orbital diagram - Valenceelectrons Orbital Diagram for Nitrogen Electron configuration of nitrogen in the excited state Atoms can jump from one orbital to another in an excited state. This is called quantum jump. The ground-state electron configuration of nitrogen is 1s 2 2s 2 2p 3. We already know that the p-subshell has three orbitals.
Solved Create the atomic orbital diagram for nitrogen. - Chegg Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so you need to show the 2p subshell and everything below it. Next, click the orbitals to add electrons (represented as arrows). For boron, you would need to show a total of five electrons.
Nitrogen, atomic structure - Stock Image - C018/3688 Nitrogen (N). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of nitrogen-14 (atomic number: 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings).
Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration Electron Configuration For Nitrogen Ion The atomic number of nitrogen is 7, the element nitrogen was discovered by a Scottish physician, Danial Rutherford. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element Nitrogen.
Molecular Nitrogen and Related Diatomic Molecules Let's look at the Lewis structure of and N2. Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2px, 2py, and 2pz). In the Lewis structure there is a triple bond between the nitrogen atoms and a non-bonding pair of electrons on each. This is consistent with the physical properties of N2. Molecular Orbitals
Nitric Oxide Molecular Orbital Diagram Something is not correct with your MO diagram. Note that in this diagram the oxygen atomic orbitals are lower in energy than the nitrogen.Jul 21, · A molecular orbital diagram that can be applied to any homonuclear diatomic molecule with two identical alkali metal atoms Nitric oxide (NO) is an example of a heteronuclear diatomic molecule.



0 Response to "38 atomic orbital diagram nitrogen"
Post a Comment