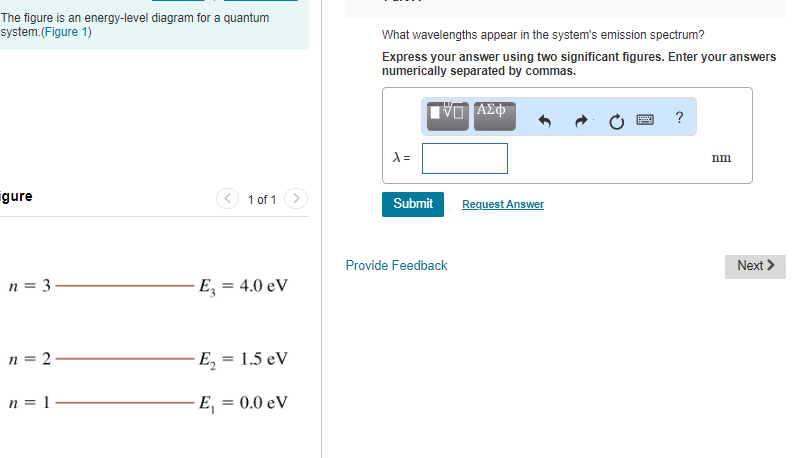
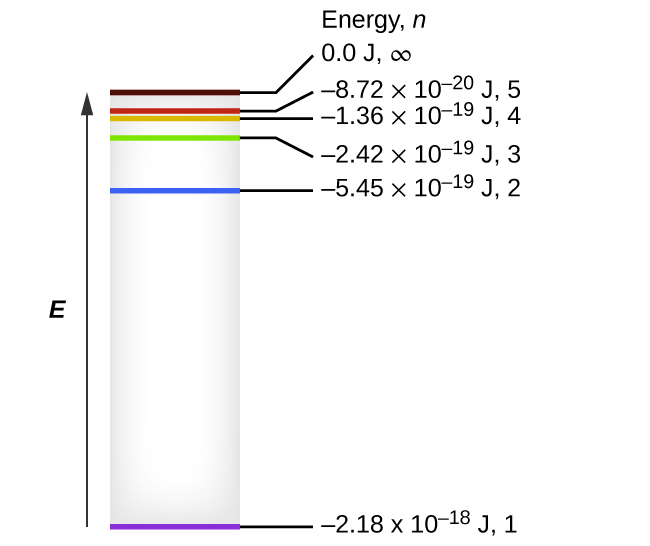
40 the figure is an energy-level diagram for a quantum system.(figure 1)
Energy Levels - an overview | ScienceDirect Topics An electronic energy level diagram showing the relative energies of the levels responsible for monomer and excimer fluorescence is shown in Figure 1. The lower energy level of the excimer produces its fluorescence with a wavelength distribution at longer values than that for the monomer fluorescence. exam 4 practice Flashcards | Quizlet The spacing between energy levels is drawn to scale. (Figure 1) P
Solved Energy 9.0 eV n = 4 6.0 eV n = 3 4.0 eV n = 2 1.0 eV Transcribed image text: Energy 9.0 eV n = 4 6.0 eV n = 3 4.0 eV n = 2 1.0 eV n = 1 Figure shows an energy-level diagram for a quantum system.
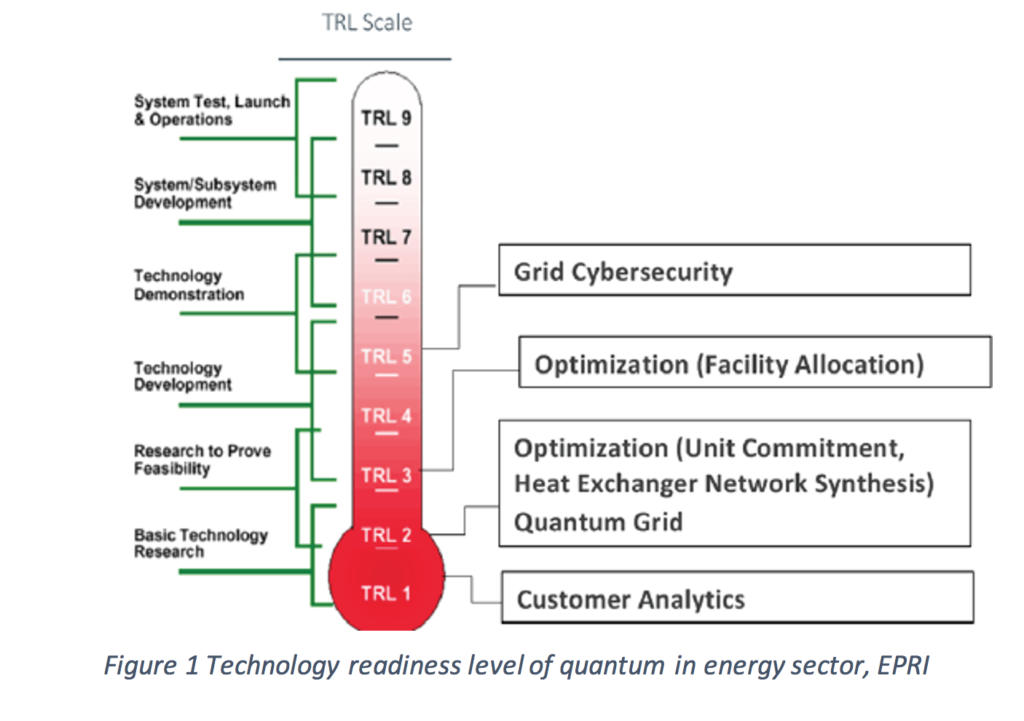
The figure is an energy-level diagram for a quantum system.(figure 1)
PDF Experiment 6: Vibronic Absorption Spectrum of Molecular Iodine energetics of the entire system is commonly depicted using a potential energy diagram. A potential energy diagram showing the first three electronic states of molecular iodine is shown in Figure 1. ΔE(I, I*) X reB I + I I + I* a EXvib(0) EBvib(0) Figure 1: A potential energy diagram for I2 1. The curve X describes the ground Answered: The figure shows some energy levels of… | bartleby Q: (Figure 1) is an energy-level diagram for a quantum system. What wavelengths appear in the system's ... A: Given for n=3 state, Energy E3 = 4.0 eV for n=2 state, Energy E2 = 1.5 eV for n=1 state, Energy E1 =... PDF Energy Diagrams I - Kansas State University ED1-1 Energy Diagrams I Goal Changes in energy are a good way to describe an object's motion. Here you will construct energy diagrams for a toy car and learn how these diagrams can be useful. This technique will prepare you for similar uses of energy diagrams in quantum physics. Introduction
The figure is an energy-level diagram for a quantum system.(figure 1). Solved The figure is an energy-level diagram for a quantum ... The figure is an energy-level diagram for a quantum system. What wavelengths appear in the system's emission spectrum? Question: The figure is an energy-level diagram for a quantum system. What wavelengths appear in the system's emission spectrum? PDF 7. Examples of Magnetic Energy Diagrams. - Columbia in energy than the two levels corresponding to µe being antiparallel to the field (D+, MS = +1/2, βe). Because the system is in a strong field it is now convenient to classify the states in terms of the individual spins on the z axis. According to the correlation diagram (Figure 15), the three low field Ten states correlate to The energy levels of a quantum particle in a box as the ... The Correspondence Principle The figure below shows the quantum and the classical probability densities for the n = 1 quantum state of a particle in a rigid box. For this ground state, the quantum and classical probability densities are quite different, so the quantum system will be very nonclassical. Solved Review Constants (Figure 1) is an energy-level - Chegg Transcribed image text: Review Constants (Figure 1) is an energy-level diagram for a quantum system Part A What wavelengths appear in the system's emission ...
Solved The figure is an energy-level diagram for a quantum Question: The figure is an energy-level diagram for a quantum system.(Figure 1) What wavelengths appear in the system's emission spectrum? Figure 4.1: Approximate energy level diagram for Sodium ... The Stark shift experienced by the Hydrogen atom with varying electric field ( E) is as shown in Figure 4.5. For each quantum number n, the splitting between energy levels increases with the ... Solved Review Curlslais Telui (Figure 1) is an energy-level Transcribed image text: Review Curlslais Telui (Figure 1) is an energy-level diagram for a quantum system. Part A What wavelengths appear in the system's ... Solved The figure is an energy-level diagram for a quantum Transcribed image text: Problem 28.39 The figure is an energy-level diagram for a quantum system.(Figure 1) n = 3 ----- E3 = 4.0 eV n = 2 ----- E2 = 1.5 eV n= 1 ----- E1 = 0.0 eV Part A What wavelengths appear in the system's emission spectrum? Express your answer using two significant figures. Enter your answers numerically separated by commas. Lambda =
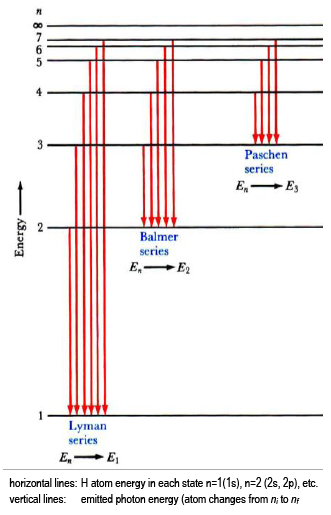
Energy Level and Transition of Electrons | Brilliant Math ... Imgur. The energy of the electron of a monoelectronic atom depends only on which shell the electron orbits in. The energy level of the electron of a hydrogen atom is given by the following formula, where. n. n n denotes the principal quantum number: E n = − 1312 n 2 kJ/mol. E_n=-\frac {1312} {n^2}\text { kJ/mol}. E n. The Quantum Harmonic Oscillator - University Physics Volume 3 A mass of 0.250 kg oscillates on a spring with the force constant 110 N/m. Calculate the ground energy level and the separation between the adjacent energy levels. Express the results in joules and in electron-volts. Are quantum effects important? PDF The Shape of the Wave Function - Kansas State University Figure 4: Diagram of a potential well and its corresponding wave function. D-1. On the figure above use a ruler to draw verticalvertical lines through the points x = -1.2, x = -0.5, x = 0, x = +0.5, and x = +1.2 D-2. Complete the table below by indicating +, 0, or - for the first four columns and Quantum energy level | Article about Quantum energy level ... Quantum transitions between energy levels are denoted in energy-level diagrams by vertical or slanted lines that connect the corresponding pairs of energy levels. Figure 1 shows radiative transitions with frequencies vik that satisfy the frequency condition hvjk = ℰ - ℰ k, where h is Planck's constant.
Quantum computing - Wikipedia Quantum simulations might be used to understand this process increasing production. Quantum annealing and adiabatic optimization. Quantum annealing or Adiabatic quantum computation relies on the adiabatic theorem to undertake calculations. A system is placed in the ground state for a simple Hamiltonian, which is slowly evolved to a more ...
Figure P28.41 is an energy-level diagram for a quantum ... Problem 39P. Figure P28.41 is an energy-level diagram for a quantum system. What wavelengths appear in the system's emission spectrum?
Figure 1: Energy level diagram for a model rotator for N ... Embed figure Energy level diagram for a model rotator for N = 4 bosons labeled by the quantum numbers (l, M ). The energy spectra for the q-deformed case and for the classical limit are shown.
PDF 4. Energy Levels - MIT OpenCourseWare Energy Levels 4.1 Bound problems 4.1.1 . Energy in Square infinite well (particle in a box) 4.1.2 ... In the limit of large quantum numbers or small deBroglie wavelength λ ∝ 1/k on average the quantum mechanical ... is negative). Notice that I set E to be a positive quantity, and the system's energy is −E. We also assume that
PDF Chapter 11 Density of States, Fermi Energy and Energy Bands 11-3 ! p k (11.6) Knowing the momentum p = mv, the possible energy states of a free electron is obtained m k m p E mv 2 2 2 1 2 2 ! (11.7) which is called the dispersion relation (energy or frequency-wavevector relation). Effective Mass In reality, an electron in a crystal experiences complex forces from the ionized atoms.
PDF Chapter 6 Nuclear Energy Levels Chapter 6—Nuclear Energy Levels 6-2 number, T, is an integer or half-integer that measures a property that results if neutron and proton coordinates were interchanged. Figure 6-1 shows these quantum numbers for each excited state in the notation J P, T.These quantum numbers are results of the basic
Solved (Figure 1) is an energy-level diagram for a quantum ... Physics questions and answers. (Figure 1) is an energy-level diagram for a quantum system. Part A What wavelengths appear in the system's emission spectrum? Express your answers in nanometers separated by commas. VO ΑΣΦ ? 828.75,310.78,407.25 nm Figure 1 of 1 Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining ...
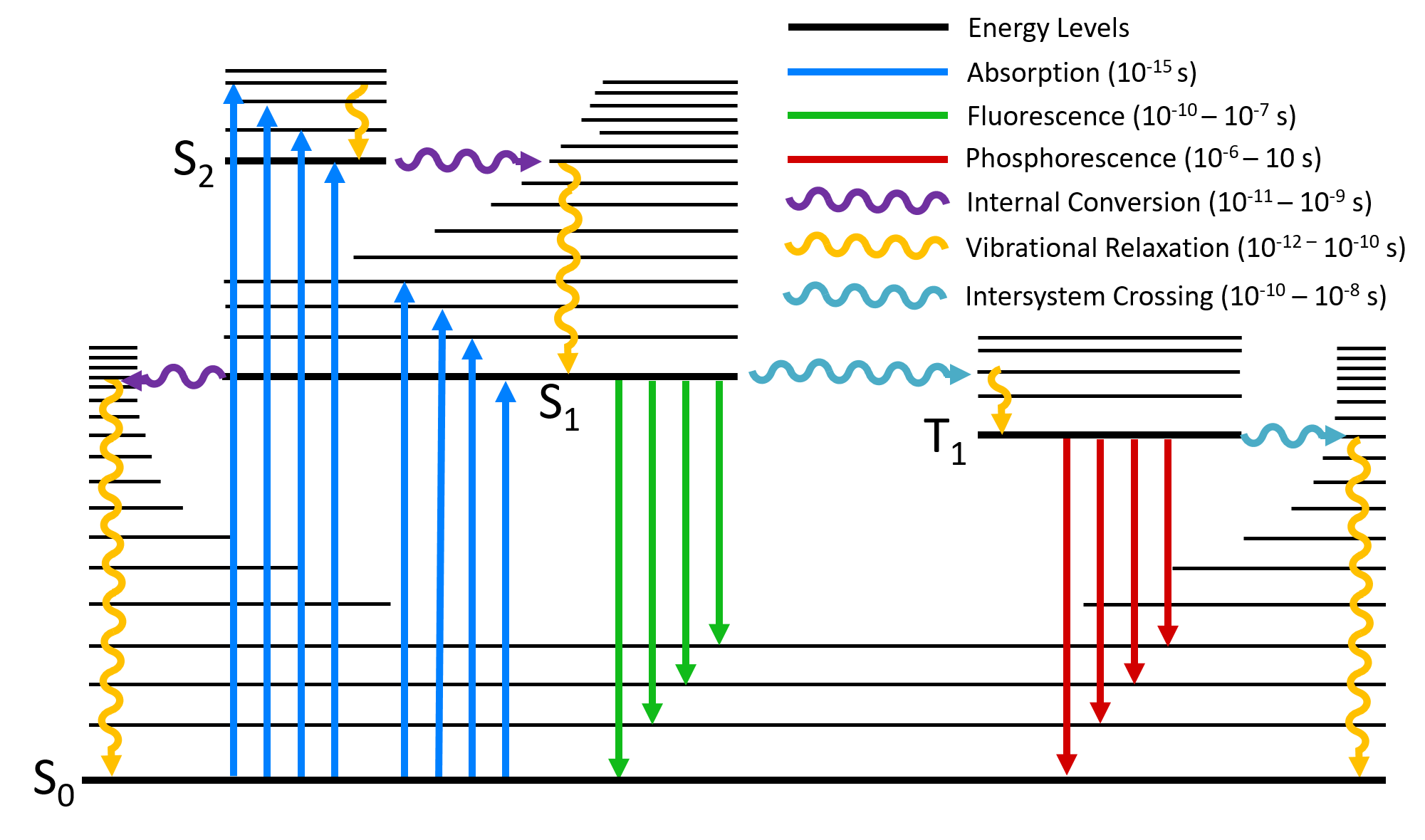
Chloroplasts and Photosynthesis - Molecular Biology of the ... The process of energy conversion begins when a chlorophyll molecule is excited by a quantum of light (a photon) and an electron is moved from one molecular orbital to another of higher energy. As illustrated in Figure 14-42, such an excited molecule is unstable and tends to return to its original, unexcited state in one of three ways:
Spin Quantum Number - an overview | ScienceDirect Topics Figure 1. The energy level diagram for an AX spin system and the allowed zero quantum (ZQ), single quantum (SQ) and double quantum (DQ) transitions. The ZQ and DQ transitions represented in the circle are forbidden according to the selection rules of NMR transitions and are indirectly detected.
Figure 1. Energy level diagram of a nucleus with a 1/2 ... Figure 1 shows the energy level diagram of a nucleus with a 1/2 spin and electron two-spin system. m S and m I are the magnetic quantum numbers for electron and nucleus, respectively. For this ...
22.1 The Structure of the Atom - Physics | OpenStax Figure 22.10 shows an energy-level diagram, a convenient way to display energy states. Each of the horizontal lines corresponds to the energy of an electron in a different orbital. Energy is plotted vertically with the lowest or ground state at the bottom and with excited states above.
Solved The figure is an energy-level diagram for a quantum Question: The figure is an energy-level diagram for a quantum system. ... eV n = 1 E_1 = 0.0 eV What wavelengths appear in the system's emission spectrum?
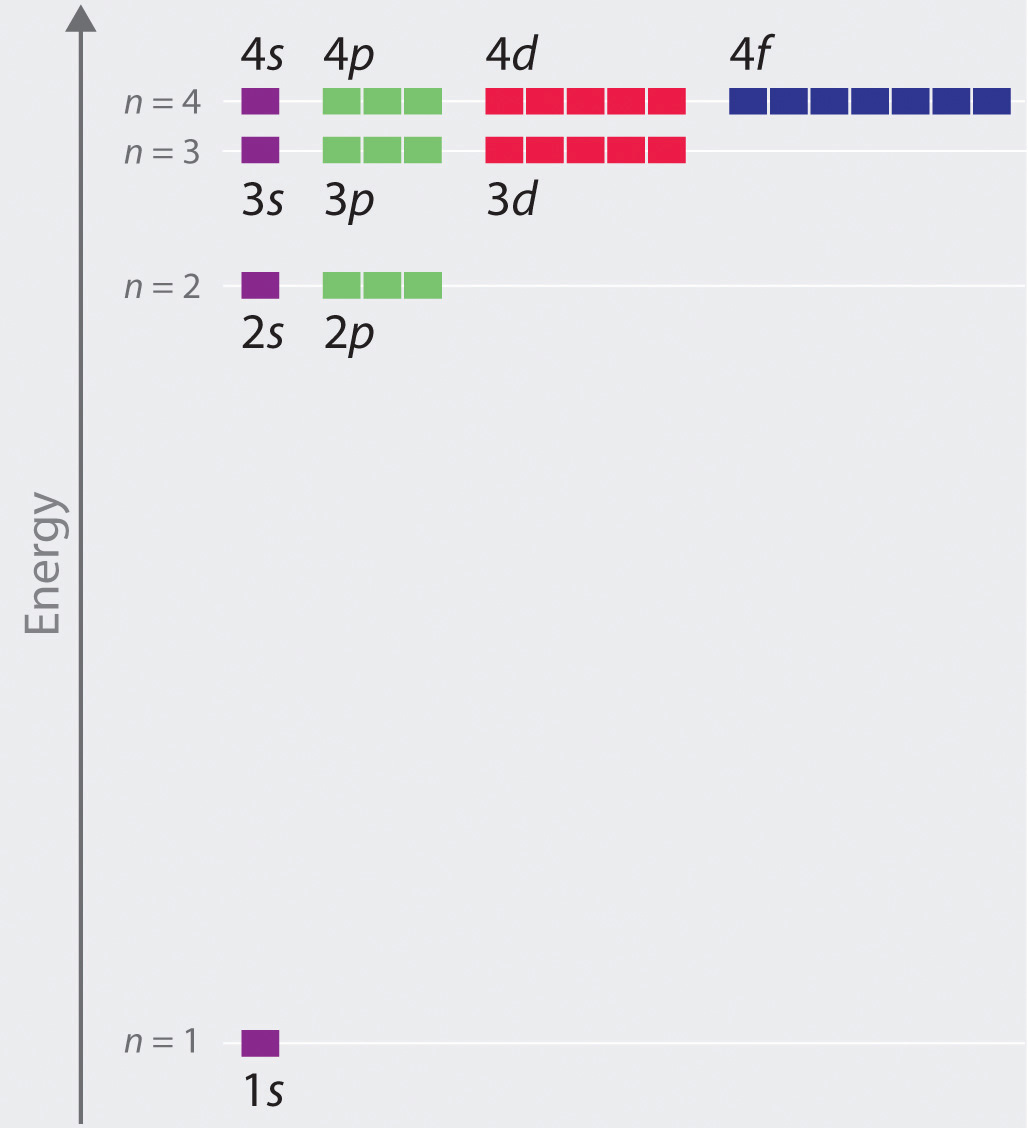
Chapter 2.5: Atomic Orbitals and Their Energies - Chemistry 003 Aug 02, 2021 · Figure 2.5.10 Orbital Energy Level Diagram for a Typical Multielectron Atom. Because of the effects of shielding and the different radial distributions of orbitals with the same value of n but different values of l, the different subshells are not degenerate in a multielectron atom.
Jablonski diagram - Chemistry LibreTexts Figure \(\PageIndex{1}\): The Foundation of a typical Jablonski Diagram. Through the use of straight and curved lines, these figures show transitions between eigenstates that occur from the exposure of a molecule to a particular wavelength of light. Straight lines show the conversion between a photon of light and the energy of an electron.
Theory of charge transport in a quantum dot tunnel ... Figure 1 (Color online) Schematic energy diagram for a quantum dot junction of concern. Γ L and Γ R denote, respectively, the tunneling rates for electrons from the source to the quantum dot and from the quantum dot to the drain (which is grounded). (a) System without bias and (b) System with forward bias. (c) System with reverse bias.Reuse ...
Quantum-coherent nanoscience | Nature Nanotechnology Nov 29, 2021 · This reduces the cooperativity of the quantum system–photon interaction, which is the key figure of merit for achieving coherent spin–photon coupling and photon-mediated spin–spin coupling ...
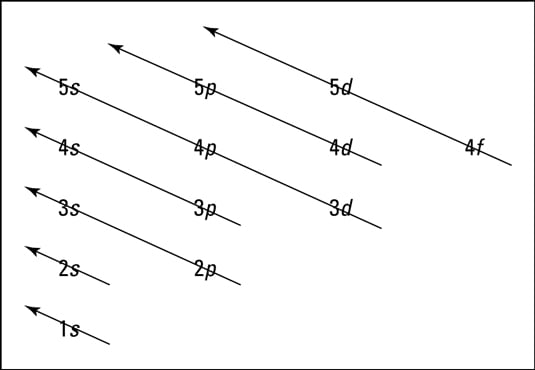
Quantum Numbers and Electron Configurations Although there is no pattern in the first four letters (s, p, d, f), the letters progress alphabetically from that point (g, h, and so on).Some of the allowed combinations of the n and l quantum numbers are shown in the figure below.. The third rule limiting allowed combinations of the n, l, and m quantum numbers has an important consequence. It forces the number of subshells in a shell to be ...
Quantum dot cellular automaton - Wikipedia Figure 3 shows the two possible minimum energy states of a quantum-dot cell. The state of a cell is called its polarization , denoted as P. Although arbitrarily chosen, using cell polarization P = -1 to represent logic “0” and P = +1 to represent logic “1” has become standard practice.
PDF Chapter 3 label the allowed energy levels. Negative values of n add nothing new because the energies in Eq (18) depends on n2. Fig. 1 shows part of the energy-level diagram for the particle in a box. The occurrence of discrete or quantized energy levels is characteristic of a bound system, that is, one conflned to a flnite region in space.
Annual Review of Physical Chemistry | Home Covers significant developments in the field of physical chemistry, including biophysical chemistry, chemical kinetics, colloids, electrochemistry, geochemistry and cosmochemistry, chemistry of atmosphere and climate, laser chemistry and ultrafast processes, the liquid state, magnetic resonance, physical organic chemistry, polymers and macromolecules, and more.
An energy-level diagram for a hypothetical atom is shown ... An energy-level diagram for a hypothetical atom is shown above. a. Determine the frequency of the lowest energy photon that could ionize the atom, initially in its ground state. b. Assume the atom has been excited to the state at -1.0 electron volt. i. Determine the wavelength of the photon for each possible spontaneous transition. ii.
Answered: (Figure 1) is an energy-level diagram… | bartleby (Figure 1) is an energy-level diagram for a quantum system. What wavelengths appear in the system's emission spectrum? Express your answers in nanometers separated by commas.
The Quantum Particle in a Box - University Physics Volume 3 The wave functions in (Figure) are sometimes referred to as the "states of definite energy." Particles in these states are said to occupy energy levels, which are represented by the horizontal lines in (Figure). Energy levels are analogous to rungs of a ladder that the particle can "climb" as it gains or loses energy.
PDF Energy Diagrams I - Kansas State University ED1-1 Energy Diagrams I Goal Changes in energy are a good way to describe an object's motion. Here you will construct energy diagrams for a toy car and learn how these diagrams can be useful. This technique will prepare you for similar uses of energy diagrams in quantum physics. Introduction
Answered: The figure shows some energy levels of… | bartleby Q: (Figure 1) is an energy-level diagram for a quantum system. What wavelengths appear in the system's ... A: Given for n=3 state, Energy E3 = 4.0 eV for n=2 state, Energy E2 = 1.5 eV for n=1 state, Energy E1 =...
PDF Experiment 6: Vibronic Absorption Spectrum of Molecular Iodine energetics of the entire system is commonly depicted using a potential energy diagram. A potential energy diagram showing the first three electronic states of molecular iodine is shown in Figure 1. ΔE(I, I*) X reB I + I I + I* a EXvib(0) EBvib(0) Figure 1: A potential energy diagram for I2 1. The curve X describes the ground



















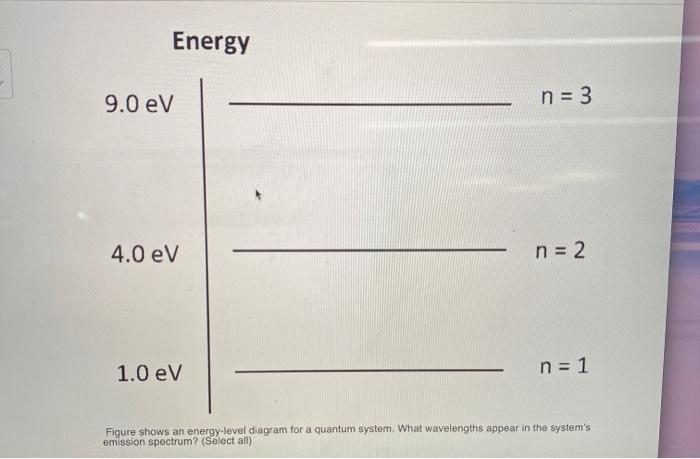
0 Response to "40 the figure is an energy-level diagram for a quantum system.(figure 1)"
Post a Comment