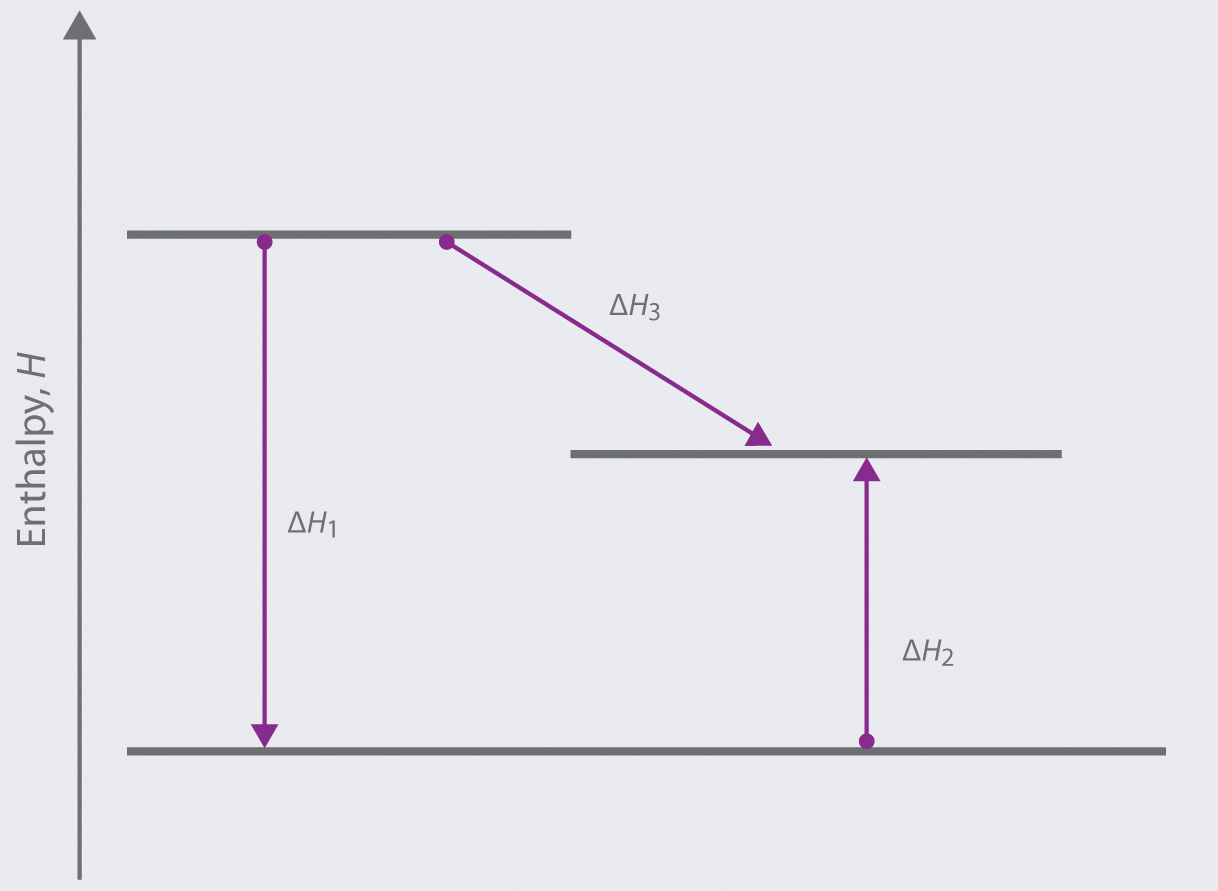
38 this diagram would represent the enthalpy changes in which of the following
AP Chemistry: Midterm Review Flashcards - Quizlet An estimate for the enthalpy change in a reaction can be made by taking the sum of the bond energies of all the bonds broken and then subtracting the sum of the bond energies of all the bonds formed. In this reaction, a total of 2 moles of C≡O bonds and 1 mole of O=O bonds are broken, while a total of 4 moles of C=O bonds are formed. How to Draw & Label Enthalpy Diagrams - Video & Lesson ... An enthalpy diagram plots information about a chemical reaction such as the starting energy level, how much energy needs to be added to activate the reaction, and the ending energy. An enthalpy...
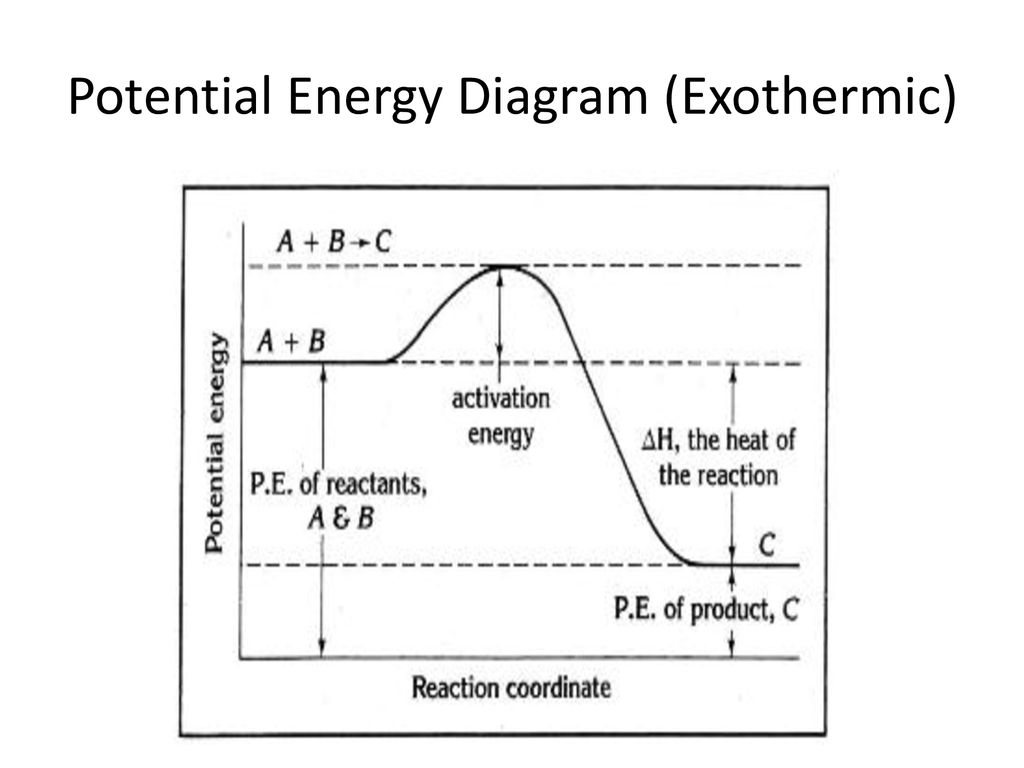
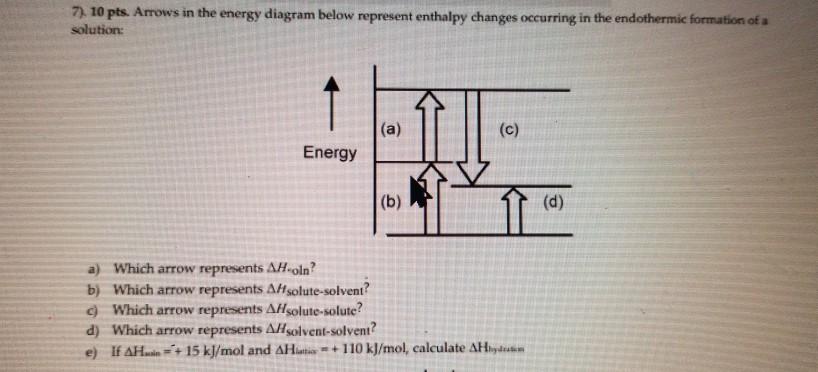
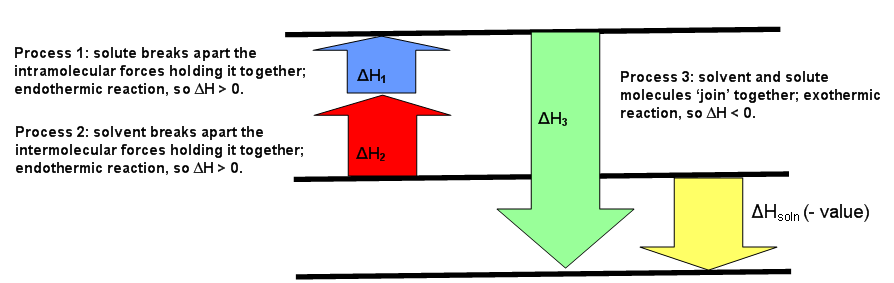
AP CHEM UNIT 7 MCQ Flashcards | Quizlet The enthalpy change for the dissolution can be evaluated only if the relative energy of these processes are known: (1) the energy required to overcome the interactions between solute particles, (2) the energy required to overcome the interactions between solvent particles, and (3) the energy released when new solute-solvent interactions are formed.
This diagram would represent the enthalpy changes in which of the following
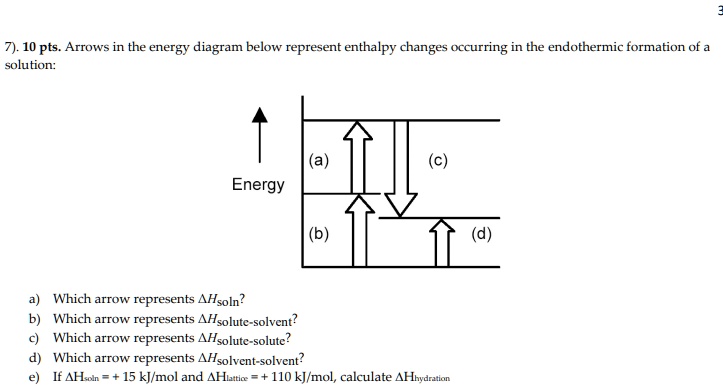
Study 16 Terms | sem 2 chem 4.06 Flashcards - Quizlet Input the variables into the equation and solve the problem. ΔH = q = mCΔT ΔH = (100g)(4.18 J/g-0C)(-1000C) ΔH=−41.8kJ The delta HΔH is negative. The reaction is exothermic. This makes sense because the water is releasing heat as it cools. Changes in state, like chemical reactions, involve changes in enthalpy Will mark as brainliest. this diagram would represent the ... Will mark as brainliest. this diagram would represent the enthalpy changes in which of the following... Questions in other subjects: Computers and Technology, 25.06.2019 17:10 Solved 9. a. If ∆H1 represents enthalpy for bond breaking ... a. If ∆H1 represents enthalpy for bond breaking and ∆H2 represents enthalpy for bond formation write down the mathematical equation for determining enthalpy of reaction. Draw an enthalpy diagram that will correctly represent the enthalpy of reaction using bond. enthalpy. b. Calculate the enthalpy change of the following reaction from the ...
This diagram would represent the enthalpy changes in which of the following. 1 Chapter 5 assignment.pdf - Name Chapter 5 Communication ... View 1 Chapter 5 assignment.pdf from CHEMISTRY CHEM 3FF3 at McMaster University. Name Chapter 5 Communication Assignment [Total marks /50] K/U = /10 1. Create a simple enthalpy diagram to represent Monkey monster Flashcards - Quizlet all of the above suspensions Arrows in the energy diagram below represent enthalpy changes occurring in the exothermic formation of a solution: ΔHsoln = enthalpy of solution ΔHsolute-solute = enthalpy change involving solute-solute interactions ΔHsolute-solvent = enthalpy change involving solute-solvent interactions Will mark as brainliest. this diagram would represent the ... Answer: 1 📌📌📌 question Will mark as brainliest. this diagram would represent the enthalpy changes in which of the following? cold pack hot pack melting solid boiling liquid - the answers to estudyassistant.com Thermodynamics Conceptual | Chemistry Quiz - Quizizz Diagram 1, because it represents a reaction that reaches equilibrium quickly after a very small amount of the reactants is consumed. Diagram 2, because it represents a reaction with a high activation energy barrier for molecules to overcome and a very slow reaction rate, even if it is thermodynamically favorable with ΔG < 0
This diagram would represent the enthalpy changes in which ... answered This diagram would represent the enthalpy changes in which of the following? boiling liquid hot pack cold pack melting solid 1 See answer Add answer + 8 pts Advertisement bmisses is waiting for your help. Add your answer and earn points. Answer 4.9 /5 51 Dejanras Answer is: hot pack. AP Classroom for Chemical Thermodynamics Flashcards | Quizlet The particle diagram does not provide a good representation for the changes in enthalpy (changes in energy) associated with the dissolution process. The reaction in which H2O (l) is decomposed into H2 (g) and O2 (g) is thermodynamically unfavorable (ΔG°>0). Chemistry 4.06 Quiz: Changes in Enthalpy Cheat Sheet ... Chemistry 4.06 Quiz: Changes in Enthalpy Cheat Sheet STUDY Flashcards Learn Write Spell Test PLAY Match Gravity What is enthalpy? Click card to see definition 👆 The heat content of a system at constant pressure Click again to see term 👆 1/5 Previous ← Next → Flip Space The given enthalpy diagram represents which of the ... Given the following reactions with their enthalpy changes, at 2 5 ∘ C, N 2 (g) + 2 O 2 (g) → 2 N O 2 (g); H = 1 6. 1 8 k c a l N 2 (g) + 2 O 2 (g) → N 2 O 4 (g); H = 2. 3 1 k c a l Calculate the enthalpy of dimerisation of N O 2 . Is N 2 O 4 apt to be stable with respect to N O 2 at 2 5 ∘ C? (1 3. 8 7 k c a l, N 2 O 4 stable only at low ...
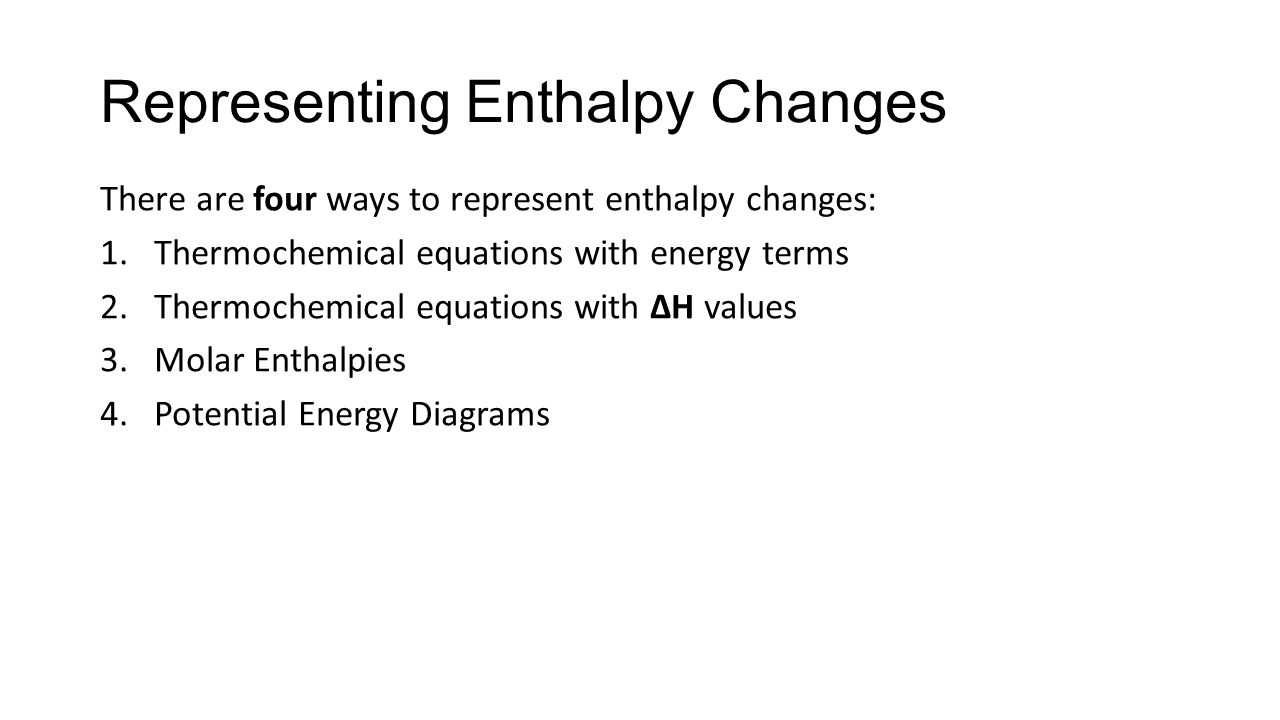
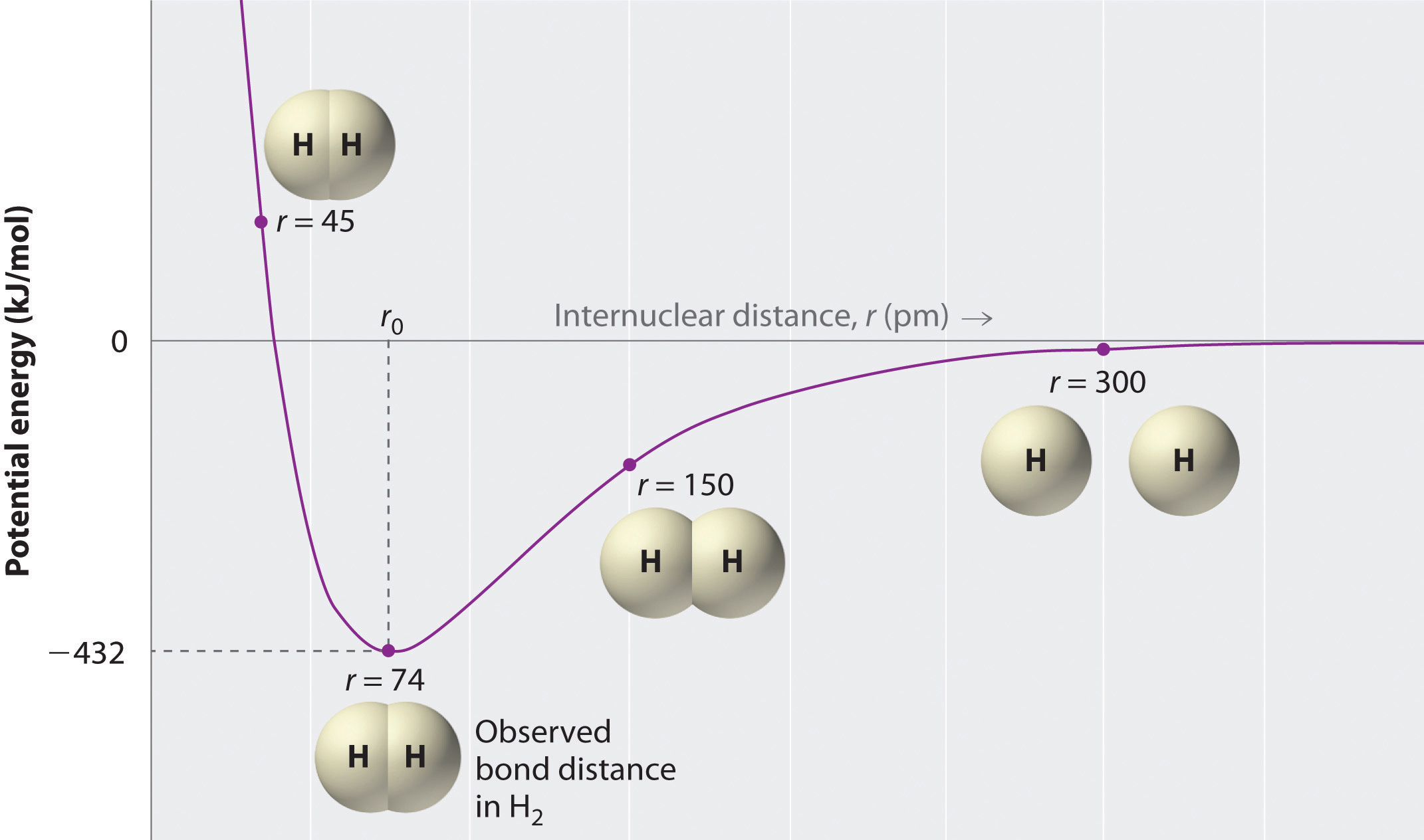
Solution: Consider the potential energy di ... - Clutch Prep Problem: Consider the potential energy diagram below:What is the change in enthalpy (ΔH ) for the reaction A → B?a. -350 kJb. 100 kJc. -100 kJd. 350 kJ FREE Expert Solution Show answer Answer: 4.06 Changes in Enthalpy Quiz Flashcards | Quizlet The latent heat in a substance has a negative value. Which situation is most likely to be true? A liquid is solidifying at the freezing point. This diagram would represent the enthalpy change in which example? liquid water freezing boiling water melting ice cold pack at a constant temperature Solved 9. a. If ∆H1 represents enthalpy for bond breaking ... a. If ∆H1 represents enthalpy for bond breaking and ∆H2 represents enthalpy for bond formation write down the mathematical equation for determining enthalpy of reaction. Draw an enthalpy diagram that will correctly represent the enthalpy of reaction using bond. enthalpy. b. Calculate the enthalpy change of the following reaction from the ... Will mark as brainliest. this diagram would represent the ... Will mark as brainliest. this diagram would represent the enthalpy changes in which of the following... Questions in other subjects: Computers and Technology, 25.06.2019 17:10
Study 16 Terms | sem 2 chem 4.06 Flashcards - Quizlet Input the variables into the equation and solve the problem. ΔH = q = mCΔT ΔH = (100g)(4.18 J/g-0C)(-1000C) ΔH=−41.8kJ The delta HΔH is negative. The reaction is exothermic. This makes sense because the water is releasing heat as it cools. Changes in state, like chemical reactions, involve changes in enthalpy
![Solved] Arrows in the Energy Diagram Below Represent Enthalpy ...](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d145_c4b5_a2f7_e9b04ca4c53b_TB4940_00_TB4940_00_TB4940_00.jpg)






















0 Response to "38 this diagram would represent the enthalpy changes in which of the following"
Post a Comment