42 which diagram is a bomb calorimeter?
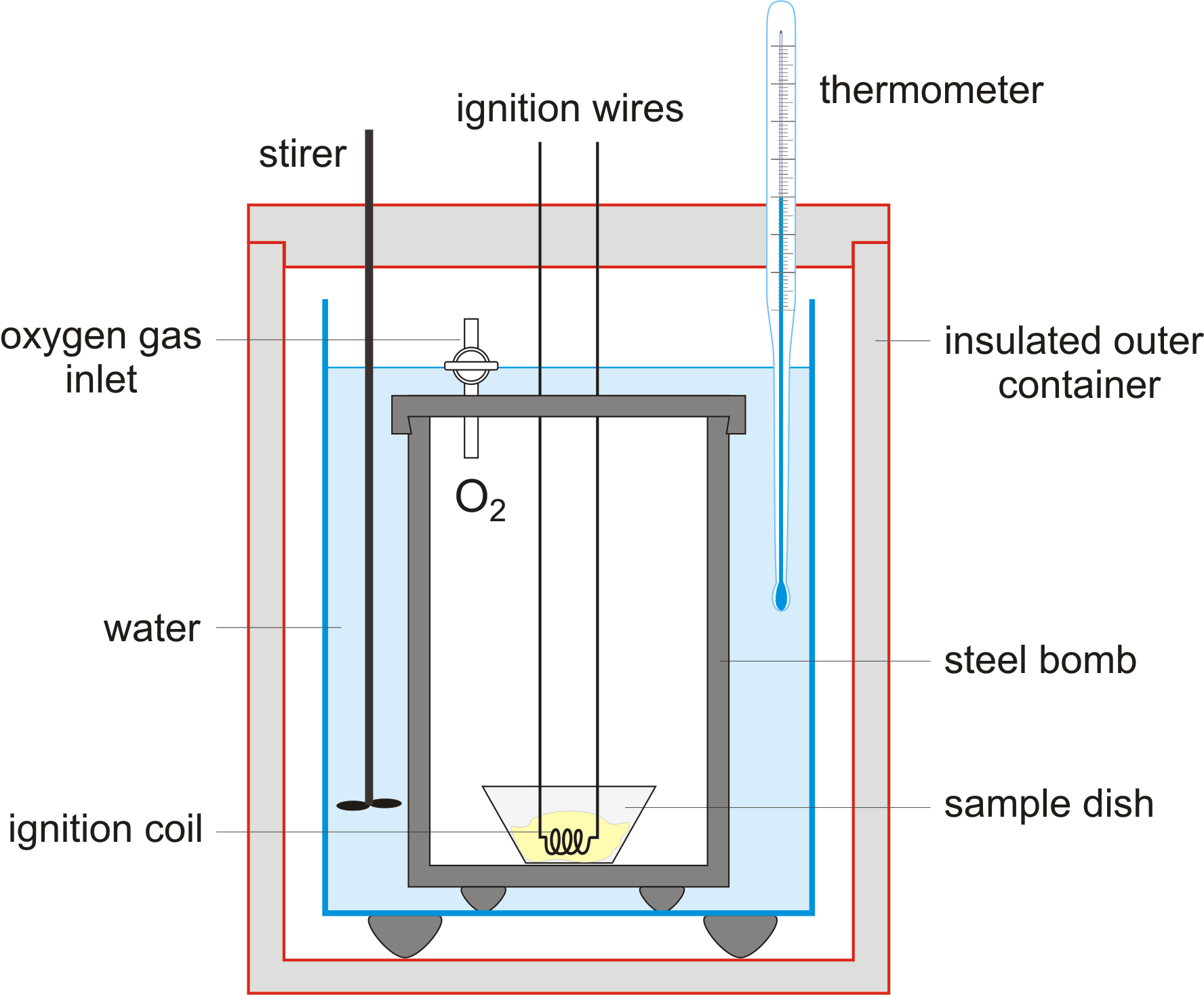
Bomb Calorimeters - Concept - Chemistry Video by Brightstorm A bomb calorimeter is used for measuring energy released in a combustion reaction. This reaction takes place in a water bath, so that the water absorbs the energy released and we can measure how much its temperature rises accordingly. bomb calorimeter. Alright so let's talk about a bomb calorimeter. A bomb calorimeters are used, they're ... Bomb calorimeter - slideshare.net Bomb Calorimeter • Bomb calorimeter is used to measure the changes in a system's internal energy due to a reaction. • The basic principle is a chemical reaction heats a quantity of water in an insulated container. • The reaction takes place inside a sealed container, called as bomb. 3. Figure: Bomb Calorimeter.
ADFS::Bluebottle.$.Files.Chemistry.MainProj - acornusers.org Diagram 1. A typical bomb. Diagram 2 - Internal representation of the steel bomb calorimeter calorimeter. The actual heat of formation is the change in heat content when one mole of compound is formed from its constituent elements in their standard physical states at 298.15K and 1 atmosphere pressure. 2.2 Calorimeter calibration
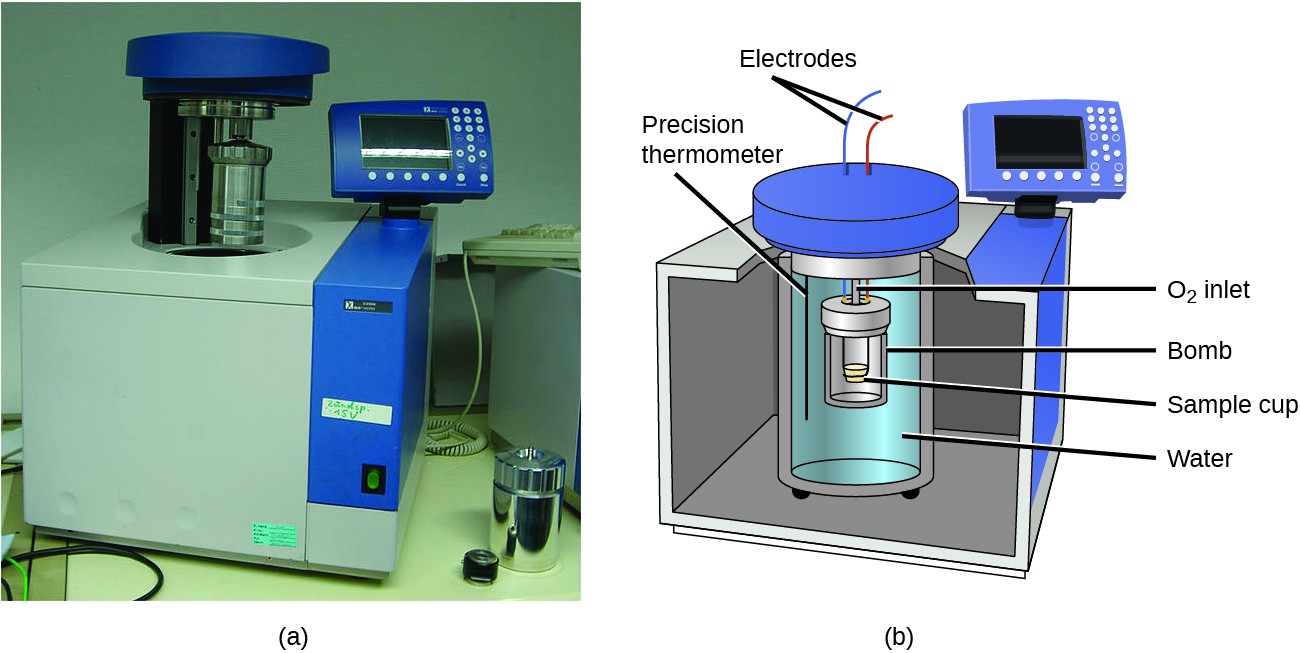
Which diagram is a bomb calorimeter?
Which diagram is a bomb calorimeter? Which diagram is a bomb calorimeter? Answers: 1 Show answers Another question on Chemistry. Chemistry, 22.06.2019 09:30. What does the mass of 0.7891 mol of ferric oxide (fe2o3) Answers: 1. Answer. Chemistry, 22.06.2019 12:30. Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae ... What is the Structure and function of bomb calorimeter? - Answers A bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of a particular reaction. Bomb calorimeters have to withstand the large pressure within the ... › technology › calorimetercalorimeter | Definition, Uses, Diagram, & Facts | Britannica calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimeters have been designed in great variety. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place, surrounded by a liquid, such as water, that absorbs the heat ...
Which diagram is a bomb calorimeter?. bomb calorimeter Flashcards | Quizlet A student attempted to measure the enthalpy change of solution of anhydrous. cobalt (II) chloride by adding 2.00 g of cobalt (II) chloride to 50.0 cm 3 of water in a. well-insulated container. A temperature rise of 1.5 °C was recorded. The student used a balance which reads to 0.01g, a 50.0 cm 3 pipette, and a. Solved Question 3 of 4 3. Label the image of the bomb - Chegg Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (33 ratings) Transcribed image text: Question 3 of 4 3. Label the image of the bomb calorimeter. :: Bomb :: Outer chamber :: Reactant :: Thermometer II :: Turbine :: Water Check Answers. Bomb calorimeter - Parts, Diagram, Working, Formula Bomb calorimeter diagram Working of Bomb Calorimeters: The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. In the calorimeter use of a strong cylindrical bomb in which combustion occurs. The bomb has two values at the top. study.com › learn › lessonBomb Calorimeter Equation & Calculation | What Does a Bomb ... Sep 27, 2021 · Bomb Calorimeter Diagram with its Parts. Bomb Calorimeter Parts and Function. The reaction of combustion takes place in the bomb, a sealed heavy-walled container. The bomb is filled with a ...
BOMB CALORIMETER - EIE Instruments PVT LTD The system will consist of the following parts and standard accessories along with instruction manual. Calorimeter vessel with bomb support & insulating base - 01; Water Jacket - 01; Combined lid for calorimeter vessel & water jacket - 01; Stirrer - 01 set Connecting lead for calorimeter bomb - 01 set Fine adjustment valve with built in pressure gauge - 01 Bomb Calorimeter - Definition, Uses, Equation - Toppr-guides Bomb calorimetry is used mainly in the scientific study of thermodynamic processes. It measures the heat of combustion produced in a chemical reaction. Also, it measures reaction enthalpy and change in enthalpy throughout the reaction. Obviously Bomb calorimeter is very essential to scientific and theoretical thermodynamic studies. Bomb Calorimeter - SlideShare Bomb Calorimeter A bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of a particular reaction. Used to measure enthalpy changes of combustion reactions at a constant volume. 6. Overview Basically, a bomb calorimeter consists of a Small cup to contain the sample Oxygen A stainless steel bomb Water ... 5.2 Calorimetry - Chemistry Bomb Calorimetry When 3.12 g of glucose, C 6 H 12 O 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases from 23.8 °C to 35.6 °C. The calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 J/°C. ... 10.4 Phase Diagrams. 10.5 The Solid State of Matter. 10.6 Lattice Structures in ...
anyflip.com › vvxce › pdgaTUTORIAL CHEMISTRY SK025 - Flip eBook Pages 1-50 | AnyFlip Jul 10, 2021 · 2. A 2.4 g sample of carbon is burnt in a calorimeter. Given that H f for CO2 is −394 kJ mol−1 and the heat capacity of the calorimeter is 10 kJ °C−1, calculate the temperature change of the calorimeter. Ans : ∆T = 7.88 oC 3. The combustion of naphthalene, C10H8, can be used to calibrate the capacity of a bomb calorimeter. Bomb Calorimetry - Hope College BOMB CALORIMETRY. 1. Purpose of Bomb Calorimetry Experiments Bomb calorimetry is used to determine the enthalpy of combustion, D comb H, for hydrocarbons: C x H Y O z (s) + (2X+Y/2-Z)/2 O 2 (g) ® X CO 2 (g) + Y H 2 O (l). Since combustion reactions are usually exothermic (give off heat), D comb H is typically negative. (However, be aware that older literature defines the "heat of combustion ... Calorimetry Flashcards - Quizlet The bomb calorimeter has a mass of 1.30 kg and a specific heat of 3.41 J/(gi°C). If the initial temperature of the calorimeter is 25.5°C, what is its final temperature? A. ... Which diagram is a bomb calorimeter? c. A 1.20-g sample of propane (C3H8) is burned in a bomb calorimeter. The temperature of the calorimeter rises from 20.0°C to 30.0°C. BYJUS BYJUS
Bomb Calorimeter - an overview | ScienceDirect Topics The bomb calorimeter consists of pressurized oxygen "bomb" (30 bar), which houses the fuel. A 10 cm fuse wire connected to two electrodes is kept in contact with the fuel inside the bomb. The oxygen bomb is placed in a container filled with 2 l of deionized water. The temperature of the water is measured by means of a precision thermocouple.
Bomb Calorimeter: Definition, Construction and Uses Sample questions based on Bomb Calorimeter. In a bomb calorimeter, a 0.88 gummy bear is burnt. The temperature rose to 21.5 °C before settling at 24.2 °C. The heat capacity of the bomb calorimeter was calculated to be 11.4 kJ/°C by the manufacturer. Calculate the amount of heat released during burning per gramme of gummy bear. Ans.
› topics › engineeringNet Calorific Value - an overview | ScienceDirect Topics In EN 14918/ISO 18125 method, about 1 g ± 0.1 of air-dry (equilibrium moisture content) analysis sample is burnt in high-pressure oxygen in a bomb calorimeter (Figure 3.12) under specified conditions. The effective heat capacity of the calorimeter is determined in calibration experiments by combustion of certified benzoic acid under similar ...
Calorimeter vs. Bomb Calorimeter - CHEMISTRY COMMUNITY Re: Calorimeter vs. Bomb Calorimeter. A bomb calorimeter is a special type of calorimeter that forces a reaction to occur under constant volume. This means that the change in internal energy will be equal to the change in q from the reaction taking place. An easy way to remember this is to imagine a reaction taking place inside the bomb ...
Coffee Cup and Bomb Calorimetry - ThoughtCo A bomb calorimeter works in the same manner as a coffee cup calorimeter, with one big difference: In a coffee cup calorimeter, the reaction takes place in the water, while in a bomb calorimeter, the reaction takes place in a sealed metal container, which is placed in the water in an insulated container. Heat flow from the reaction crosses the ...
Bomb Calorimeter Equation & Calculation | What Does a Bomb Calorimeter ... What Does a Bomb Calorimeter Measure? A bomb calorimeter measures the heat released or absorbed during a burning reaction, expressed in quantities of calories or Joules. Calories and Joules are units of department of energy. Therefore, a bomb calorimeter calorimeter measures the amount of energy ( in terms of heat
quizlet.com › 202434015 › chm-115-learnsmart-chapterCHM 115 LearnSmart Chapter 6 Flashcards - Quizlet - The direction of the arrow in an enthalpy diagram indicates whether a reaction is exothermic or endothermic - In an endothermic reaction, the reactants will be at the bottom of the enthalpy diagram Match each type of calorimeter with the thermochemical property that it measures
The Difference Between a Coffee Cup Calorimeter and a Bomb Calorimeter In a bomb calorimeter, the reaction takes place in a sealed metal container, which is the bomb vessel. The bomb vessel is then placed in an insulated adiabatic chamber (polystyrene insulation in some models). The other major difference is that the bomb vessel is a sealed high-pressure vessel. The bomb vessel, with the sample inside, is inflated ...
› examplesLearn Thermodynamics - Example Problems 1D-1 - Bomb Calorimeter; 1D-2 - Thermodynamic Cycles in Normal Life; 1D-3 - Identifying Types of Equilibria; 1D-4 - Identifying a Quasi-Equilibrium Process; Lesson E - Temperature, Pressure & Volume. 1E-1 - Pressure Measurement Using a Multi-Fluid Manometer; 1E-2 - Pressure Gage and Manometer Readings; 1E-3 - Pressure in a Tank Using a Complex ...
Bomb Calorimeter - an overview | ScienceDirect Topics Figure 5-9 is a schematic diagram of an oxygen bomb calorimeter, which is given this name because samples are combusted in pure oxygen at an elevated pressure of about 25 bars.A carefully weighed sample is put into the platinum crucible and then the bomb is sealed and placed into an insulated water bath. The bomb is repeatedly flushed with O 2 (g) to remove all air.
byjus.com › physics › calorimeterCalorimeter - Definition, Uses, Types, Application, Diagram Say in a calorimeter a fixed amount of fuel is burned. The vessel is filled with water, and the fuel is burned, leading to the heating of water. Heat loss by the fuel is equal to the heat gained by the water. This is why it is important to insulate the calorimeter from the environment; to improve the accuracy of the experiment.
Calorimetry Chemistry Tutorial - AUS-e-TUTE A bomb calorimeter can be used to measure heat content of foods and fuels. A schematic diagram of a typical bomb calorimeter is shown below: The "bomb" is the inner steel container in which the sample will be combusted rapidly and completely using oxygen gas at a pressure of about 25 atm (≈ 2533 kPa).
Bomb Calorimetry - Hope College where the mass of the burned fuse is determined by weighing the fuse before and after firing the bomb. 5. B. Nonadiabaticity of calorimeter. A bomb calorimeter is only approximately adiabatic. In reality, there is a small heat leak through the dewar (q calorimeter ¹ 0) and the stirrer does work on the calorimeter (w calorimeter ¹ 0).
What is bomb calorimeter diagram? - Brainly.in Bomb calorimeter is used to measure heat during chemical reactions. It is also called constant volume calorimeter. It is made up of a stainless steel bomb, water, stirrer, a thermometer and a small cup having oxygen. The reactant is electrically ignited inside the stainless steel bomb. This in return raises the temperature of the whole system ...
How does a bomb calorimeter work? BOMB CALORIMETERS EXPLAINED. Combustion Calorimeters measure the heat released from a combustible solid-liquid substance. This is done by weighing a precise measure of the sample substance into a crucible, placing the crucible inside a "bomb" (a sealed metal cylinder called a vessel), filling the vessel with oxygen and igniting the substance.
Bomb Calorimeter: Definition, Equation & Example - Study.com A 13.36 g sample of an unknown hydrocarbon undergoes combustion in a bomb calorimeter with a calorimeter constant of 18.5 kJ / degree C. If the calorimeter increases in temperature from 15.75 to 25.52
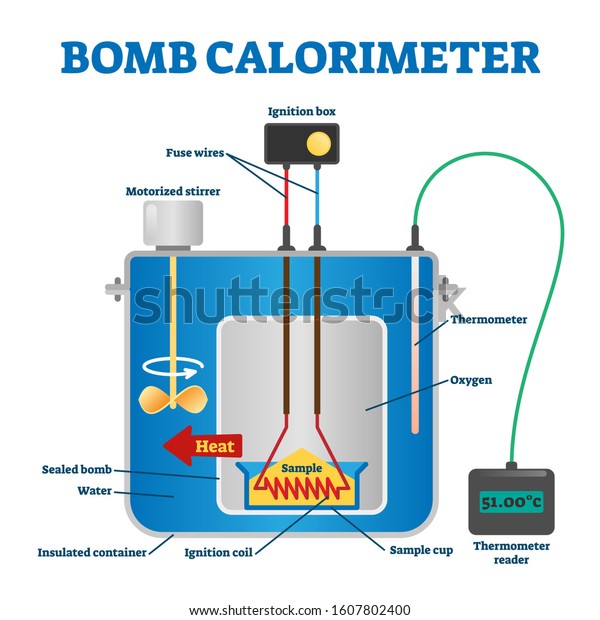




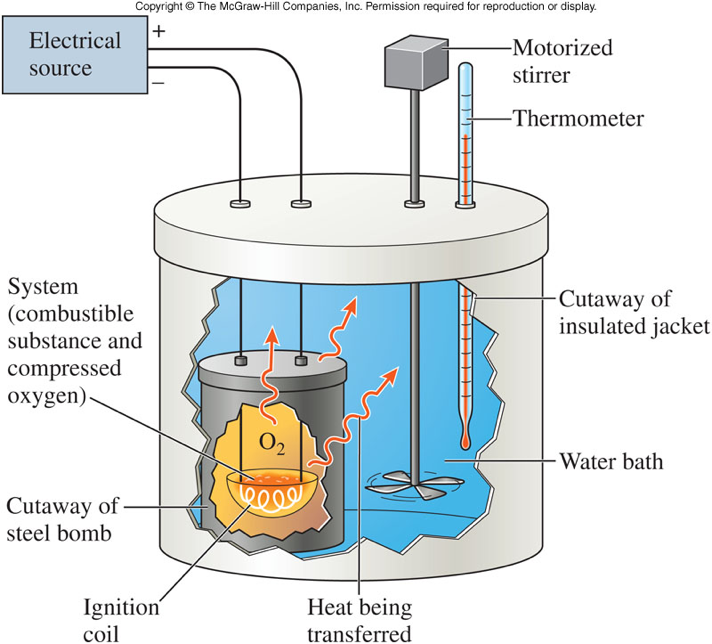



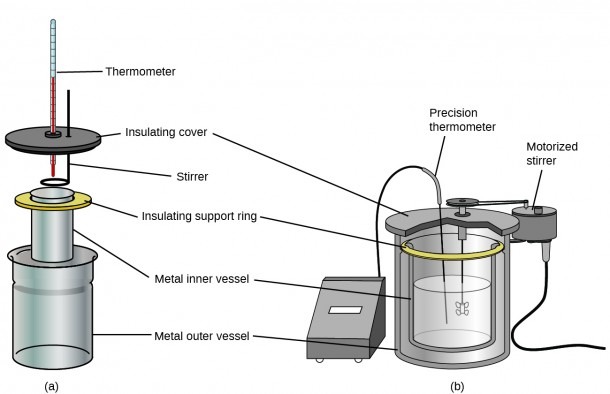
0 Response to "42 which diagram is a bomb calorimeter?"
Post a Comment