44 orbital diagram for beryllium
Beryllium - Wikipedia Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal.It is a divalent element that occurs naturally only in combination with other elements to form minerals. Notable gemstones high in beryllium include beryl (aquamarine, emerald) and chrysoberyl.It is a relatively rare element in the universe, usually ... Orbital energy diagram for beryllium? | Socratic The AO diagrams are on the left or right. The MO diagram is the diagram as a whole. If you want to draw the excited state, draw a 2p AO a bit further above and move one 2s electron (from the highest-occupied AO) into the 2p AO (the lowest-unoccupied AO). LINEAR COMBINATION OF SIGMA ns AOs
OneClass: A molecular orbital diagram for beryllium (Be) is shown. Will ... 28 Nov 2020 A molecular orbital diagram for beryllium (Be) is shown. Will beryllium exist as a molecule according to molecular orbital theory? Yes No Because: a. Electrons in bonding orbitals make the species more stable and electrons in antibonding orbitals make the species less stable. b.

Orbital diagram for beryllium
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12 ... Beryllium oxide | BeO - PubChem Beryllium oxide | BeO | CID 14775 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. National Institutes of Health. National Library of Medicine. National Center for Biotechnology Information. PubChem ... What does the molecular orbital scheme of beryllium chloride and ... Beryllium is a somewhat fascinating element since it is the only member of the second group that behaves somewhat non-metalish and, e.g. forms a somewhat covalent chloride and a covalent hydride. In an answer to a different and unrelated question, I tried guessing what the bonding picture of B e C l X 2 would be.
Orbital diagram for beryllium. What is the orbital diagram for helium? - nsnsearch.com What is the orbital diagram? Orbital diagrams are a pictorial description of electrons in an atom.In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest energy orbital available. Beryllium Orbital Diagram - Learnool As mentioned above, the electron configuration of beryllium is 1s 2 2s 2. Hence, draw the blank orbital diagram of beryllium upto 2s subshell as follows: In the above orbital diagram, the box represents an orbital. Each orbital has a capacity of two electrons. And the arrows (↑↓) are drawn inside the box to represent electrons. Electron Configuration for Beryllium (Be) - TerpConnect Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining 2 electrons for Be go in the 2s orbital. Therefore the Be electron configuration will be 1s 2 2s 2. Molecular Orbital (MO) Diagram of Be2 - YouTube Molecular Orbital Diagram for Beryllium Dimer (Be2)Fill from the bottom up, with 4 electrons total.Bonding Order is 0, meaning it does not bond, and it is di...
Which orbital notation represents an atom of beryllium in Orbital notation represents an atom of beryllium in the ground state is .. Beryllium atom: Beryllium is the fourth element with a total of 4 electrons.Beryllium atoms have 4 electrons and the shell structure is 2.2. The ground state electron configuration of ground state gaseous neutral beryllium is .The ground state of an electron, the energy level it normally occupies, is the state of lowest ... Lithium Orbital Diagram - Learnool In the above orbital diagram, the box represents an orbital. Each orbital has a capacity of two electrons. And the arrows (↑↓) are drawn inside the box to represent electrons. Now 1s 2 indicates that the 1s subshell has 2 electrons. So draw two arrows in the 1s box showing two electrons as follows: Chem4Kids.com: Beryllium: Orbital and Bonding Info Once one shell is full, the next electron that is added has to move to the next shell. So... for the element of BERYLLIUM, you already know that the atomic number tells you the number of electrons. That means there are 4 electrons in a beryllium atom. Looking at the picture, you can see there are two electrons in shell one and two in shell two. Orbital Diagram For Beryllium The orbital diagram for beryllium is shown here. The electron configuration is 1s 2 2s 2. The fourth electron is placed in the 2s orbital. The energy required to pair the first 2s electron is less than the energy required to place the electron into the 2p orbital. Our beryllium page has over facts that span different quantities.
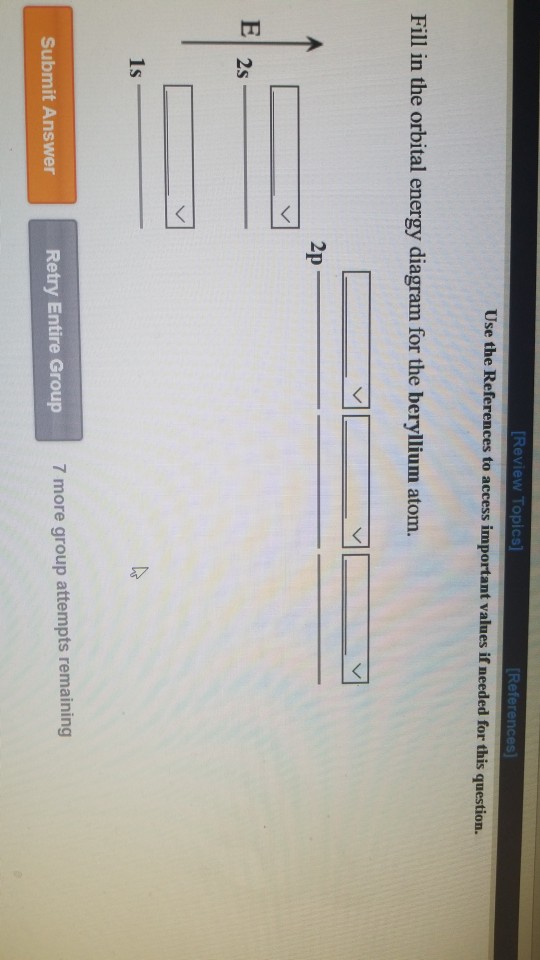
Solved Be sure to answer all parts. Using the periodic - Chegg Enter the orbital diagram below. Include blank spaces for empty orbitals. (select) (select) (select) (select) (select) 1s 2s 2p ; Question: Be sure to answer all parts. Using the periodic table, write the electron configuration and orbital diagram of beryllium. Enter the electron configuration below. Enter the orbital diagram below. Electron configuration for Beryllium (element 4). Orbital diagram Beryllium electron configuration. ← Electronic configurations of elements. Electronic configuration of the Beryllium atom: 1s 2 2s 2. Reduced electronic configuration Be: [He] 2s 2. Below is the electronic diagram of the Beryllium atom Distribution of electrons over energy levels in the Be atom. 1-st level (K): 2. Draw the orbital energy diagram for Beryllium. - Vedantu It states that each orbital must be singly occupied before the filling of electrons takes place. Now, in the case of Beryllium, to sketch its orbital energy diagram we first need to write its electronic configuration. Atomic number of Beryllium = 4 Electronic configuration of Beryllium = 1 s 2 2 s 2 Molecular Orbital (MO) Diagram of Polyatomic molecules Beryllium ... Molecular Orbital (MO) Diagram of Polyatomic molecules Beryllium dihydride (BeH2) and Water (H2O) 25,719 views Oct 4, 2020 460 Dislike Share Save Edmerls 52.8K subscribers Subscribe In polyatomic...
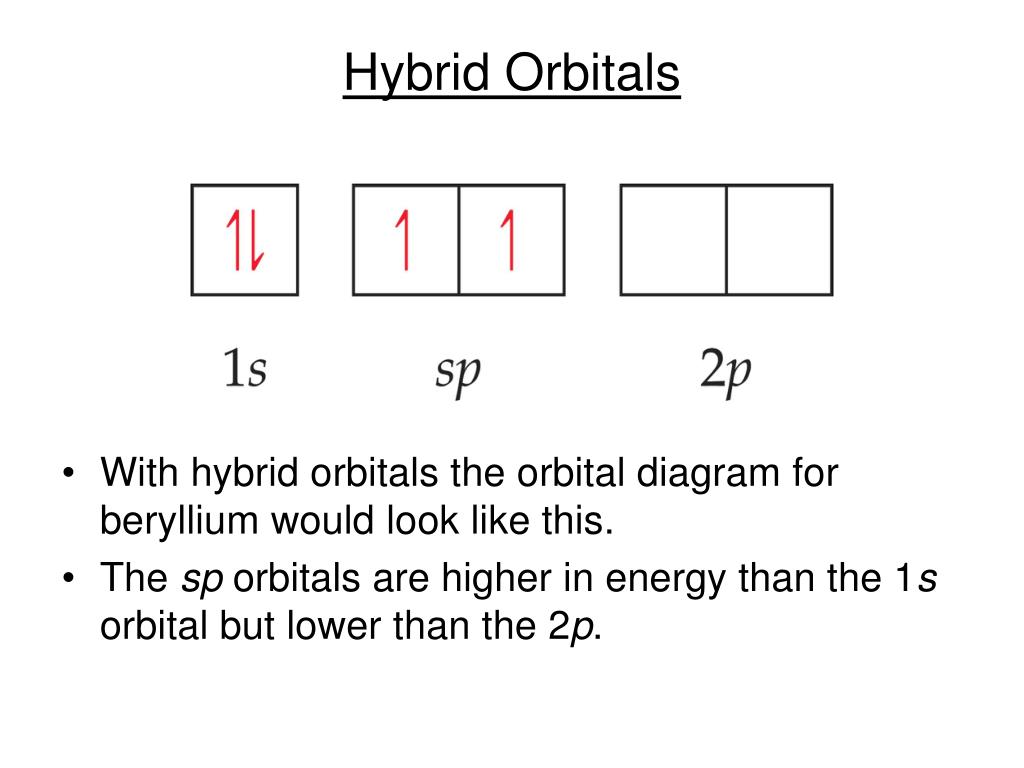
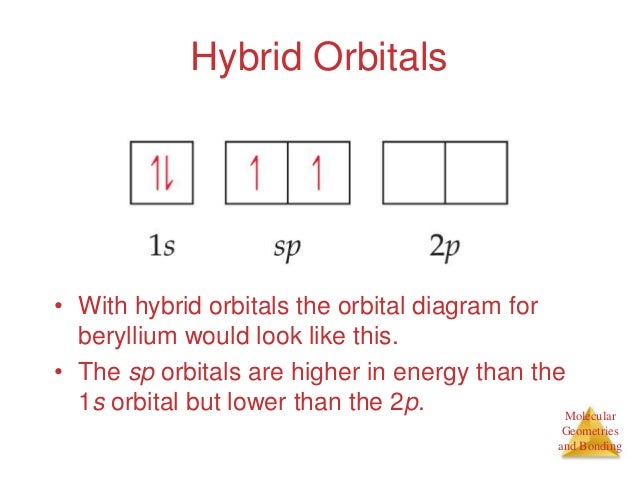
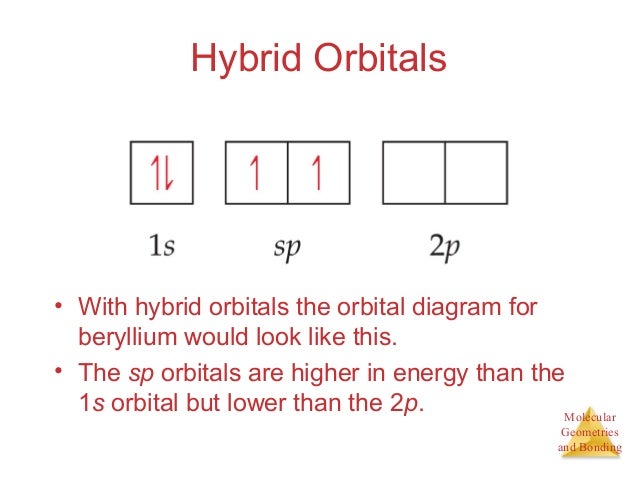
BeH2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity The sp hybrid orbital of the beryllium atom overlap with the 1s atomic orbital of the hydrogen atom, which is shown in the orbital diagram of the beryllium hydride molecule as follows: Hence, the VBT method also leads to the sp hybridization of the Beryllium atom in Beryllium hydride with linear geometry. BeH2 Polarity
Orbital filling diagrams | The Cavalcade o' Chemistry The orbital filling diagram for helium The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital.
Beryllium Orbital Diagram As a solid. Dec 29, · An advanced molecular orbital diagram of BeH2 (beryllium hydride) for the inorganic or physical chemistry student. For the most part, beryllium oxide is produced as a white amorphous powder, sintered into larger shapes. Impurities, like carbon, can give a variety of colours to the otherwise colourless host crystals.
Lithium(Li) electron configuration and orbital diagram To write the orbital diagram of lithium (Li), you have to do the electron configuration of lithium. Which has been discussed in detail above. Lithium orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital.
Beryllium(Be) electron configuration and orbital diagram Beryllium excited state electron configuration and orbital diagram The p-subshell has three orbitals. The orbitals are p x, p y, and p z. We already know that each orbital can have a maximum of two electrons. Therefore, the electron configuration of beryllium (Be*) in excited state will be 1s 2 2s 1 2p x1.
How to draw Bohr diagram for Beryllium(Be) atom - Topblogtenz Steps to draw the Bohr Model of Beryllium atom 1. Find the number of protons, electrons, and neutrons in the Beryllium atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electrons are the negatively charged particles that orbit the nucleus of an atom
PDF Electronic band structure of beryllium oxide - RSPhys - ANU Electronic band structure of beryllium oxide V A Sashin ‡, M A Bolorizadeh ... with band structure calculations performed within the full potential linear muffin-tin orbital approximation. Our experimentalbandwidths of 2.1) 0.2 eV and 4.8 0.3 eV for the oxygen ... thereby allowing them to construct the complete energy-level diagram. They ...
BeCl2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and ... The molecular orbital diagram of the BeCl2 molecule is drawn by the combination of Beryllium atomic orbitals and chlorine group orbitals. As there are two chlorine atoms and hence, first they combine to form group orbitals. The electronic configuration of Cl is [Ne] 3s23p5.
SOLVED:Use orbital diagrams to explain how the beryllium chloride ... What kind of hybrid orbitals does beryllium use in this molecule? VIDEO ANSWER: To explain the bonding of beryllium chloride, we'll start with the atomic orbital's of beryllium, Brilliant has two electrons in its two s orbital promotion can occur so that we can get to half field Limited Time Offer
Beryllium Orbital diagram, Electron configuration, and Valence electrons The orbital diagram for Beryllium is drawn with 2 orbitals. The orbitals are 1s and 2s. The Beryllium orbital diagram contains 2 electrons in 1s orbital and the remaining 2 electrons in 2s orbital. The orbital diagram for a ground-state electron configuration of the Beryllium atom is as follows - What is the electron configuration of the Be2+ ion?
What does the molecular orbital scheme of beryllium chloride and ... Beryllium is a somewhat fascinating element since it is the only member of the second group that behaves somewhat non-metalish and, e.g. forms a somewhat covalent chloride and a covalent hydride. In an answer to a different and unrelated question, I tried guessing what the bonding picture of B e C l X 2 would be.
Beryllium oxide | BeO - PubChem Beryllium oxide | BeO | CID 14775 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. National Institutes of Health. National Library of Medicine. National Center for Biotechnology Information. PubChem ...
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12 ...





0 Response to "44 orbital diagram for beryllium"
Post a Comment