39 orbital diagram for oxygen
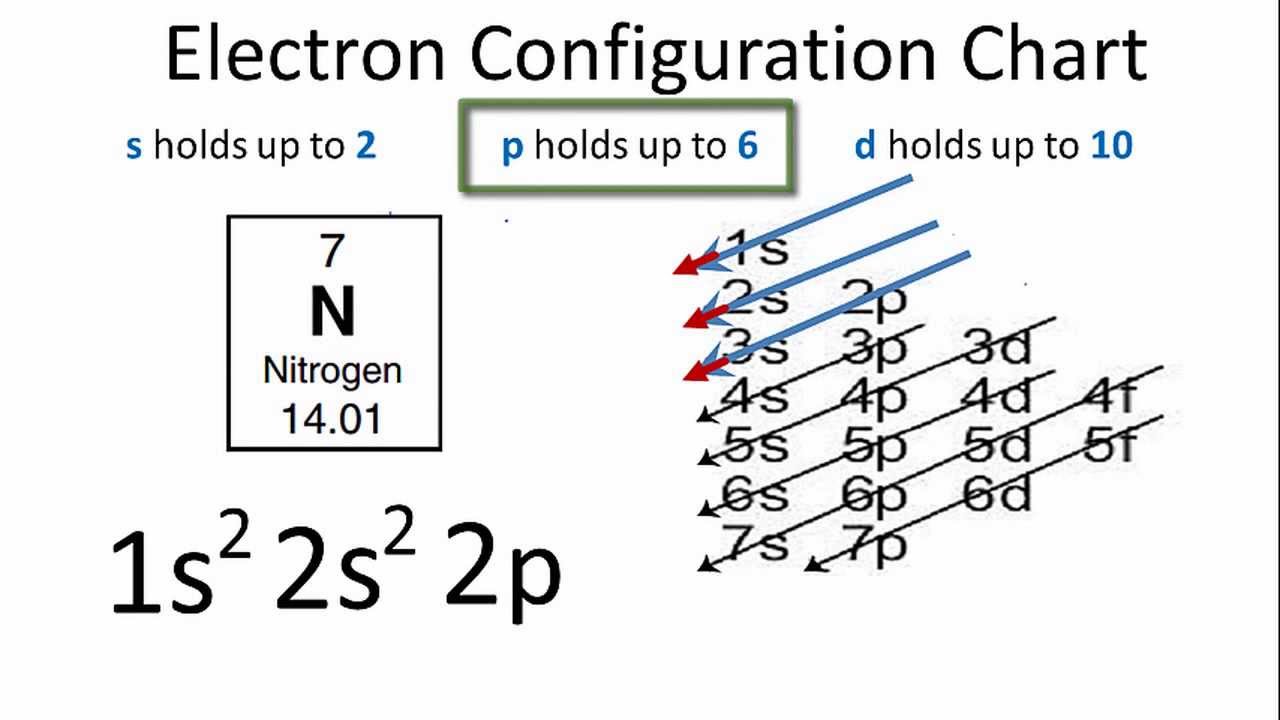
PDF Lecture 2 Mar 11: Oxygen General - MIT Oxygen is extremely electronegative (3.5) only exceeded by F, and possesses a significant ionization energy (1313.5 kJ mol-1). Atomic O has an electronic configuration 1s22s22p4. This gives rise to the diatomic molecular orbital diagram: O2 ground state: There are two low-lying singlet excited states: How do yo write the orbital diagram for oxygen? | Socratic The electron configuration for oxygen is: 1s22s22p4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps! Answer link
Oxygen Orbital Diagram - Learnool #3 Draw Orbital Diagram of Oxygen. Before drawing the orbital diagram, you should know the three general rules. Aufbau principle - electrons are first filled in lowest energy orbital and then in higher energy orbital; Pauli exclusion principle - two electrons with the same spin can not occupy the same orbital;
Orbital diagram for oxygen
Atomic Structure for Oxygen (O2) | Best Guide (With Diagrams) An Oxygen atom has: 8 protons. 8 electrons. 8 neutrons. To know more about the atomic structure of oxygen, you need to learn about the electronic configuration. The electronic configuration shows the distribution of electrons in an atom. And, it can be shown in two ways: In the form of shells. In the form of orbitals. Ozone Molecular Orbital Diagram Figure Molecular Orbital Energy-Level Diagram for \ (\pi\) Each oxygen atom in ozone has 6 valence electrons, so O 3 has a total of Let's look at the molecular orbital diagram of ozone. We'll use the hybrid orbital approximation. Each oxygen atom combines its 2s, 2pz and 2py orbitals to make .Colby College Molecular Orbitals for Ozone Purpose ... valenceelectrons.com › scandium-electron-configurationScandium(Sc) electron configuration and orbital diagram Orbital diagram for scandium Scandium excited state electron configuration. Atoms can jump from one orbital to another orbital in the excited state. This is called quantum jump. The ground state electron configuration of scandium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 4s 2. This electron configuration shows that the last shell of the scandium atom ...
Orbital diagram for oxygen. Oxygen Orbital diagram, Electron configuration, and Valence electron The orbital diagram for Oxygen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Oxygen orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and the rest four electrons in the 2p orbital. An orbital diagram for a ground-state electron configuration of Oxygen atom is shown below- recorder.butlercountyohio.org › search_records › subdivisionWelcome to Butler County Recorders Office Copy and paste this code into your website. Your Link Name Sodium(Na) electron configuration and orbital diagram The atomic number of an element is the number of electrons and protons in that element. That is, the number of electrons and protons in the sodium atom is eleven. The sodium electron configuration is 1s 2 2s 2 2p 6 3s 1. The active atomic mass of the sodium atom is 22.98976928. Sodium is an alkali metal. Give the orbital diagram for an atom of oxygen. | Study.com The orbital diagram is also one way of representing the electron configuration. Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer The...
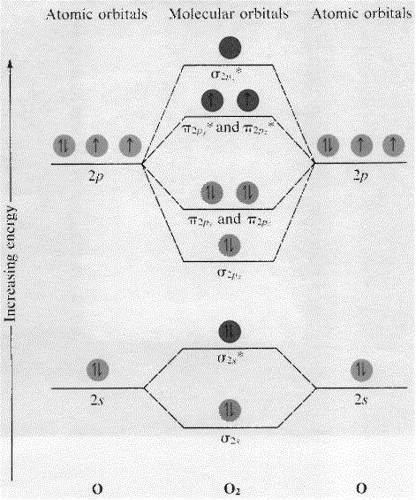
How to draw Bohr diagram for Oxygen(O) atom - Topblogtenz Here, we will draw the Bohr diagram of the Oxygen atom with some simple steps. Steps to draw the Bohr Model of Oxygen atom 1. Find the number of protons, electrons, and neutrons in the Oxygen atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*... What is the orbital diagram of oxygen? - letto.jodymaroni.com Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. The Pauli Exclusion Principle says that only two electrons can fit into an single orbital. Beside above, how many orbitals does oxygen have? Each oxygen bonds to the other with its 1s, 2s, and 2p orbitals ... en.wikipedia.org › wiki › Molecular_orbital_diagramMolecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
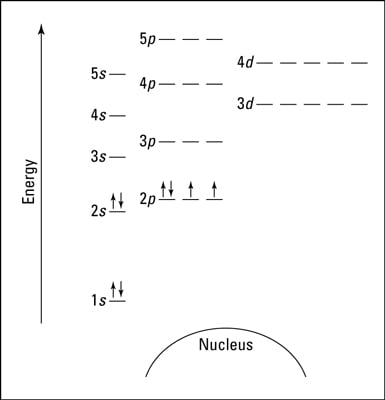
Oxygen - Wikipedia Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds.Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. valenceelectrons.com › nitrogen-electron-configurationNitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen Electron configuration of nitrogen in the excited state. Atoms can jump from one orbital to another in an excited state. This is called quantum jump. The ground-state electron configuration of nitrogen is 1s 2 2s 2 2p 3. We already know that the p-subshell has three orbitals. PDF Draw the orbital diagram for oxygen - Hoa Lavender The order of the levels filled looks like this: 1S, 2S, 2P, 3S, 3P, 4S, 3D, 4P, 5S, 4D, 5P, 6S, 4F, 5D, 6P, 7S, 5F, 6D and 7P Remember this pattern, probably the easiest, is referring to the periological table and remember where every orbital block falls to logically deduce this pattern. What is the orbital diagram of oxygen? What is the orbital diagram of oxygen? In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be 1s22s22p4.
en.wikipedia.org › wiki › Lewis_structureLewis structure - Wikipedia Count valence electrons. Nitrogen has 5 valence electrons; each oxygen has 6, for a total of (6 × 2) + 5 = 17. The ion has a charge of −1, which indicates an extra electron, so the total number of electrons is 18. Connect the atoms by single bonds. Each oxygen must be bonded to the nitrogen, which uses four electrons—two in each bond.
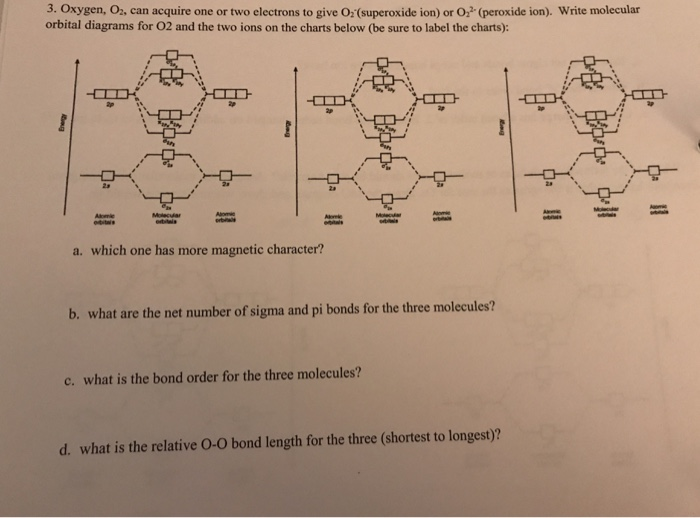
Molecular Orbital (MO) Diagram for O2(-) - YouTube When two oxygen atoms overlap, the sigma(2p) molecular orbital is LOWER in energy than the pi(2p) orbitals. This different from Nitrogen, where it's the othe...
7.7 Molecular Orbital Theory - Chemistry Fundamentals From this diagram, calculate the bond order for [latex]\ce{O2}[/latex]. How does this diagram account for the paramagnetism of [latex]\ce{O2}[/latex]? Show Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 7.7.12. Each oxygen atom contributes six electrons, so the diagram appears as shown in Figure 7.7.15 ...
periodictableguide.com › orbital-diagram-of-allOrbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
8 - Drawing Molecular Orbital Diagrams — Flux Science Well, s-p mixing doesn't occur with diatomic oxygen, creating a molecular orbital diagram like the first in this article. This is because, as more electrons are added to a system, the higher the energy becomes, due to their electrostatic repulsion. If the energy of the 2s and 2p orbitals are too far apart, mixing won't occur.
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired ... Each oxygen atom contributes six electrons, so the diagram appears as shown in [link]. The molecular orbital energy diagram for O 2 predicts two unpaired electrons. We calculate the bond order as Oxygen's paramagnetism is explained by the presence of two unpaired electrons in the (, )* molecular orbitals. Check Your Learning
What is the orbital diagram of oxygen? In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be 1s22s22p4. Click to see full answer
valenceelectrons.com › oxygen-electron-configurationOxygen(O) electron configuration and orbital diagram The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram. Electron configuration of oxygen atom through orbital. Atomic energy shells are subdivided into sub-energy levels.
What is the orbital diagram of oxygen? In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be 1s22s22p4. Click to see full answer
valenceelectrons.com › scandium-electron-configurationScandium(Sc) electron configuration and orbital diagram Orbital diagram for scandium Scandium excited state electron configuration. Atoms can jump from one orbital to another orbital in the excited state. This is called quantum jump. The ground state electron configuration of scandium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 4s 2. This electron configuration shows that the last shell of the scandium atom ...
Ozone Molecular Orbital Diagram Figure Molecular Orbital Energy-Level Diagram for \ (\pi\) Each oxygen atom in ozone has 6 valence electrons, so O 3 has a total of Let's look at the molecular orbital diagram of ozone. We'll use the hybrid orbital approximation. Each oxygen atom combines its 2s, 2pz and 2py orbitals to make .Colby College Molecular Orbitals for Ozone Purpose ...
Atomic Structure for Oxygen (O2) | Best Guide (With Diagrams) An Oxygen atom has: 8 protons. 8 electrons. 8 neutrons. To know more about the atomic structure of oxygen, you need to learn about the electronic configuration. The electronic configuration shows the distribution of electrons in an atom. And, it can be shown in two ways: In the form of shells. In the form of orbitals.







0 Response to "39 orbital diagram for oxygen"
Post a Comment