38 lewis dot diagram for pocl3
Bromate ion (BrO3-) lewis dot structure, molecular ... - Topblogtenz BrO3- is a polar molecule because of its the distorted shape that leads to some net dipole moment in it. The overall formal charge in BrO3- is -1. The molecular geometry of BrO3- is trigonal pyramidal. Total 16 lone pairs electrons and 10 bonded pairs electrons present in BrO3- lewis structure. How to Draw Lewis Dot Structures | Chem Lab For anions add a number of electrons equal to the negative charge. For cations subtract a number of electrons equal to the positive charge. 4. Place one electron pair between each pair of adjacent atoms (as determined from the framework found in step 2) to form a single bond. 5.
POCl3 Lewis Structure: How to Draw the Dot Structure for POCl3 Let's do the POCl3 Lewis structure. On the periodic table, Phosphorus in group 5 or 15, 5 valence electrons; 6 for the Oxygen; and then 7 for Chlorine, we do have three Chlorines. If we add them up: 5 plus 6, 11; plus 21: 32 valence electrons. We'll take and put the Phosphorus at the center. It is the least electronegative.

Lewis dot diagram for pocl3
PCl3 Lewis Structure: How to Draw the Dot Structure for PCl3 Let's do the Lewis structure for PCl3. Phosphorus, on the periodic table, is in group 5, it has 5 valence electrons. Chlorine, group 7, but we have three of those so we have 5 plus 7 (times 3 is 21) is 26 valence electrons. We'll put the Phosphorus in the center and then we'll put the Chlorines around it, just like that. SOLVED:Give Lewis dot structures and sketch the shapes of the following ... The loose structure s Tetra Hydra for option B, it is Try Gunnel by perimeter Octa Headroom for option C on option D It is tetrahedron. ... Give Lewis dot structures and sketch the shapes of the following: (a). I3- (b) POCl3 (c). ClOF2+ Get the answer to your homework problem. Try Numerade Free for 7 Days. Continue. Aadit S. University of New ... Lewis Structure for PO3 3- - UMD For the PO3 3- Lewis structure we first count the valence electrons for the PO3 3- molecule using the periodic table. Once we know how many valence electrons there are in PO3 3- we can distribute them around the central atom and attempt to fill the outer shells of each atom. There are a total of 26 valence electrons in the PO3 3- Lewis structure.
Lewis dot diagram for pocl3. HCN Lewis Structure & Molecular Geometry - What's Insight Hydrogen Cyanide (HCN) is a colourless, flammable, and poisonous liquid. HCN Lewis structure comprises three different atoms: Hydrogen, carbon, and nitrogen. It is a polar molecule with bond angles of 180 degrees. HCN is used in electroplating, mining, and as a precursor for several compounds. Name of molecule. Lewis Structure of POCL3? - CHEMISTRY COMMUNITY Re: Lewis Structure of POCL3? You would have to leave the P-O bond as a double bond with two lone pair electrons because when you calculate the formal charge, it would be zero at every atom. If you left it as a single bond with three lone pairs, Oxygen would have a FC of -1 and P would have a formal charge of 1. PDF Chapter 1,2 Carbon Compounds, Chemical Bonds, Lewis Structures ... Valence electrons can be indicated by dots (electron-dot formula or Lewis structures) but this is time-consuming"! The usual way to indicate the two electrons in a bond is to use a ... Correct Lewis Structure Incorrect Lewis Structures . Chapter 1! 10! It is often necessary to use double or triple bonds to satisfy the octet rule: ... POCl3 (Phosphorus Oxychloride): Structure, Properties, & Uses Structure of POCl 3. POCl 3 molecules feature 3 phosphorus-chlorine single bonds and one phosphorus-oxygen double bond. This molecule assumes a tetrahedral shape. The structure of a POCl 3 molecule is illustrated below. Structure of POCl3. It can be noted that the P=O bond is much stronger than the P-Cl bond.
HEY MATE Q lewis dot structure of POCL3 - Brainly.in Answer: Step 1: Connect the atoms with single bonds. The less electronegative is the phosphorous atom. Hence, the P atom is going to be the central atom. Recall that electronegativity decreases as we move away from the fluorine atom in the periodic chart.Step 2: Calculate the # of electrons in π bonds (multiple bonds) using formula (1): POCl3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle ... Here we will look at the Lewis structure of POCl3 to further determine its molecular geometry, bond angle, and shape. Phosphorus atom will be placed in the center as it is the least electronegative out of all the atoms. Rest all chlorine and oxygen atoms will be placed around this central atom. PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram The lewis structure of PCl3 can be explained as follows : To draw the lewis structure, first of all, we need to sum up the valence electrons of all the atoms. Here, Phosphorous = 5 valence electrons Chlorine = 7 valence electrons 3* Cl = 7*3 = 21 So total valence electrons = 26 Now we need to consider a central atom. POCl3 Lewis structure, Molecular geometry, Hybridization, Polar or ... The Lewis diagram is more stable when the formal charge of an atom is lower. Steps for drawing Lewis dot structure of POCl3 Count the total number of valence electrons present on each atom of POCl3 The total number of valence electrons of POCl3 molecule is 32. Chlorine lies on the group 17th in the periodic table and contains a valency of 7.
P2h4 Lewis Structure - example 2 drawing the lewis structure for pocl3 ... P2h4 Lewis Structure - 17 images - h2 lewis structure how to draw the dot structure for h2 youtube, chemistry chemical bonding 25 of 35 lewis structures phosphoric, ch2chch2 lewis structure youtube, can some one create a lewis dot diagram for the following yahoo answers, ClF3 Lewis Structure, Molecular Geometry, Hybridization, and Polarity In ClF3 Formal charge of Cl = Valence electrons (7) - 0.5*Bonding electrons (6) - Lone pair of electrons (2*2) = 7 - 3 - 4 = 0. Formal charge of each F atom = Valence electrons (7) - 0.5*Bonding electrons (2) - Lone pair of electrons ( 2*3) = 7 - 1 - 6 = 0. Therefore, we have got the most perfect Lewis Structure of ClF3. Solved In the POCl3 molecule, the P atom is the central - Chegg Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. tha …. View the full answer. Transcribed image text: In the POCl3 molecule, the P atom is the central atom. Draw a Lewis diagram for POCl3 in which all atoms have a formal charge of zero. Lewis Structure Of Pocl3 - bonding general concepts, draw the lewis ... Lewis Structure Of Pocl3. Here are a number of highest rated Lewis Structure Of Pocl3 pictures upon internet. We identified it from reliable source. Its submitted by dealing out in the best field. We take this nice of Lewis Structure Of Pocl3 graphic could possibly be the most trending topic when we portion it in google pro or facebook.
pof3 lewis structure - Gerstenfield pof3 lewis structuresvetlana invitational 2022 Consultation Request a Free Consultation Now. hisc hose nozzle parts. pof3 lewis structure. June 7, 2022 bye my irresistible love novel ...
Phosphoryl chloride - Wikipedia POCl 3 reacts with water to give hydrogen chloride and phosphoric acid : O=PCl 3 + 3 H 2 O → O=P (OH) 3 + 3 HCl Intermediates in the conversion have been isolated, including pyrophosphoryl chloride, P 2 O 3 Cl 4. [8] Upon treatment with excess alcohols and phenols, POCl 3 gives phosphate esters : O=PCl 3 + 3 ROH → O=P (OR) 3 + 3 HCl
Phosphorus trifluoride (PF3) lewis dot structure ... - Topblogtenz In this article, we will discuss Phosphorous trifluoride (PF3) lewis dot structure, molecular geometry, electron geometry, hybridization, polar or nonpolar, its bond angle, etc. "Phosphorus trifluoride is similar to carbon monoxide in that it is a gas which strongly binds to iron in hemoglobin, preventing the blood from absorbing oxygen.".
OneClass: Phosphoryl chloride, POCl3, has the skeleton structure. Write ... Write (a) A Lewis structure for POCl3 following the octet rule. Calculate . Get the detailed answer: Phosphoryl chloride, POCl3, has the skeleton structure. Write (a) A Lewis structure for POCl3 following the octet rule. Calculate 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ...
Solved Two Lewis structures are shown for the POCl3 - Chegg a.) Complete the Lewis diagrams by adding all NONZERO formal charges to all atoms. show any resedence structures. just draw the lewis dot structure with the non zero formal charges around the atoms. b.) Based on formal charges, which; Question: Two Lewis structures are shown for the POCl3 molecule in the following window. a.)
POCl3 Lewis Structure (Phosphoryl Chloride) - YouTube Hi Guys !Today in this video, we will determine the Lewis dot structure for Phosphoryl Chloride, having a chemical formula of POCl3. It comprises one Phospho...
PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and ... Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds.
How to draw PCl3 Lewis Structure? - Science Education and Tutorials Key Points To Consider When Drawing The PCl3 Electron Dot Structure. A three-step approach for drawing the PCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the PCl3 molecule, to add valence electrons around the phosphorus atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get ...
How to Draw the Lewis Structure for POCl3 - YouTube A step-by-step explanation of how to draw the POCl3 Lewis Dot Structure (Phosphoryl chloride).For the POCl3 structure use the periodic table to find the tota...
How to draw Lewis dot structures | POCl3 Phosphorous oxychloride Fig.1: Connect the atoms of the POCl3 molecule with single bonds Step 2: Calculate the # of electrons in π bonds (multiple bonds) using formula (1): Where n in this case is 5. Where V = (7 +5 + 7 + 6 + 7 ) = 32 , V is the number of valence electrons of the POCl3.molecule. Therefore, P = 6n + 2 - V = 6 * 5 + 2 - 32 = 0 So, there is no double bond.
Phosphorus oxychloride | POCl3 - PubChem Phosphorus oxychloride | POCl3 or Cl3OP | CID 24813 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological ...
Lewis Structure for PO3 3- - UMD For the PO3 3- Lewis structure we first count the valence electrons for the PO3 3- molecule using the periodic table. Once we know how many valence electrons there are in PO3 3- we can distribute them around the central atom and attempt to fill the outer shells of each atom. There are a total of 26 valence electrons in the PO3 3- Lewis structure.
SOLVED:Give Lewis dot structures and sketch the shapes of the following ... The loose structure s Tetra Hydra for option B, it is Try Gunnel by perimeter Octa Headroom for option C on option D It is tetrahedron. ... Give Lewis dot structures and sketch the shapes of the following: (a). I3- (b) POCl3 (c). ClOF2+ Get the answer to your homework problem. Try Numerade Free for 7 Days. Continue. Aadit S. University of New ...
PCl3 Lewis Structure: How to Draw the Dot Structure for PCl3 Let's do the Lewis structure for PCl3. Phosphorus, on the periodic table, is in group 5, it has 5 valence electrons. Chlorine, group 7, but we have three of those so we have 5 plus 7 (times 3 is 21) is 26 valence electrons. We'll put the Phosphorus in the center and then we'll put the Chlorines around it, just like that.
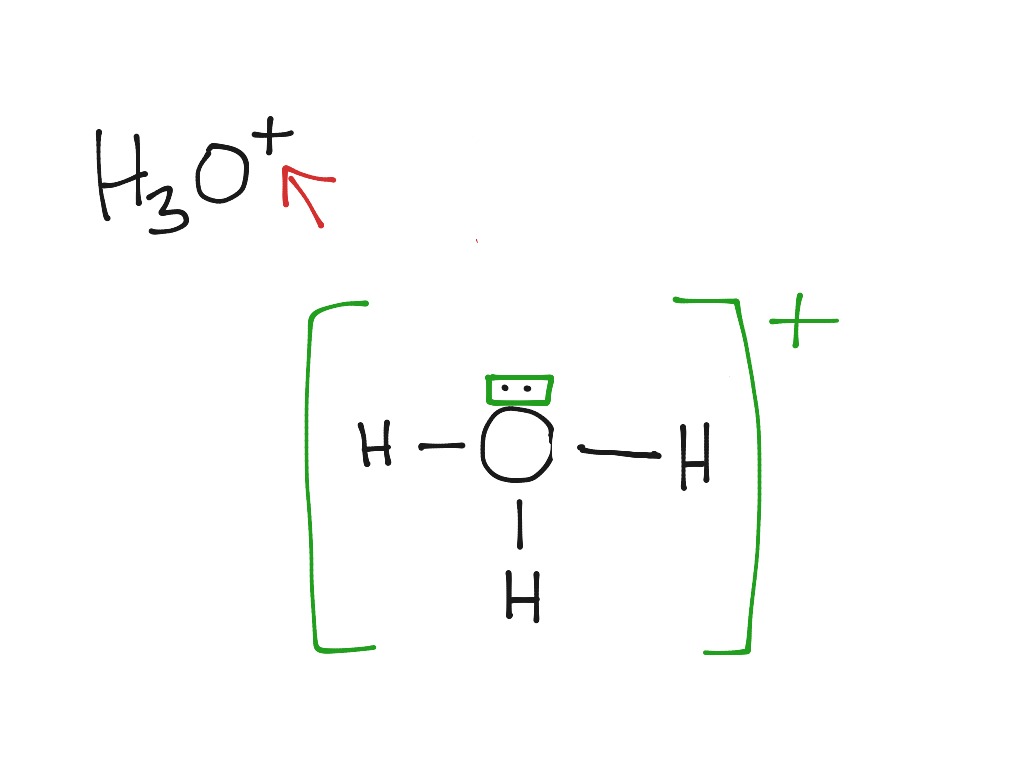











0 Response to "38 lewis dot diagram for pocl3"
Post a Comment