40 orbital diagram of carbon before sp3 hybridization
What does the atomic orbital diagram of carbon look like before sp3 ... Get the detailed answer: What does the atomic orbital diagram of carbon look like before sp3 hybridization? ... What does the atomic orbital diagram of carbon look like before sp3 hybridization? Answer +20. Watch. 1. answer. 0. watching. 308. views. For unlimited access to Homework Help, a Homework+ subscription is required. 5 - Bonding Orbitals and Hybridization — Flux Science Before we get deep into how orbital diagrams help to explain hybridization, I'd like to go summarize the last two arcs in a few sentences (yes, really). ... More specifically, each "lobe" of the hybrid sp 3 orbitals are separated by 109.5° angles to avoid electron repulsion. Therefore, ... Like carbon, it will also form sp 3 hybrid ...
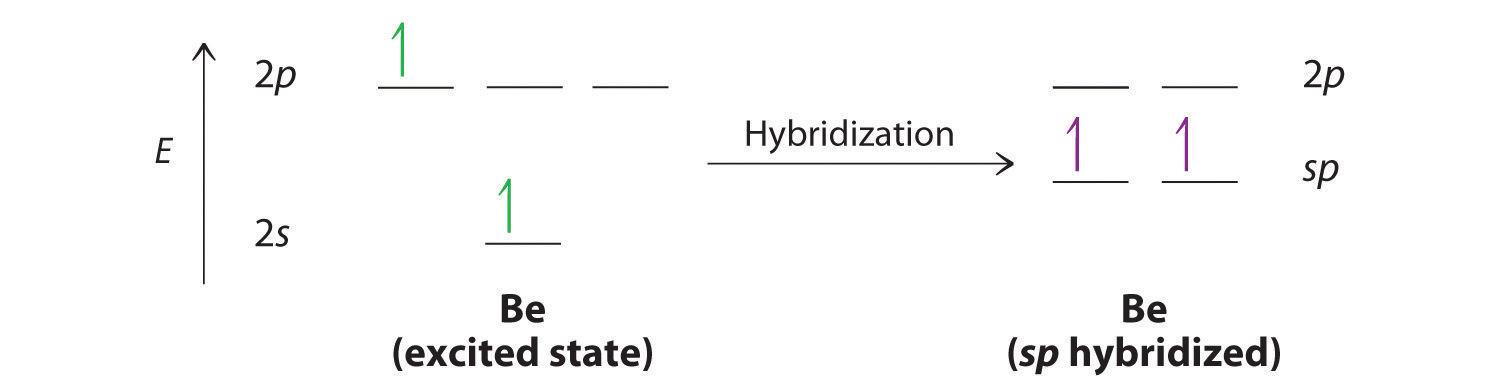
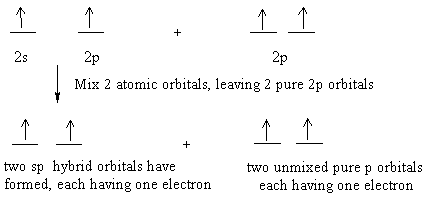
SOLVED:Draw orbital diagrams (boxes with arrows in them) to ... - Numerade Problem 30 Easy Difficulty Draw orbital diagrams (boxes with arrows in them) to represent the electron configurations of carbon before and after sp hybridization. Answer Carbon has an electron configuration of 1 s 2 2 s 2 2 p 2. During sp hybridization, one s and one p orbital of carbon combine to form two sp hybrid orbitals. View Answer Discussion
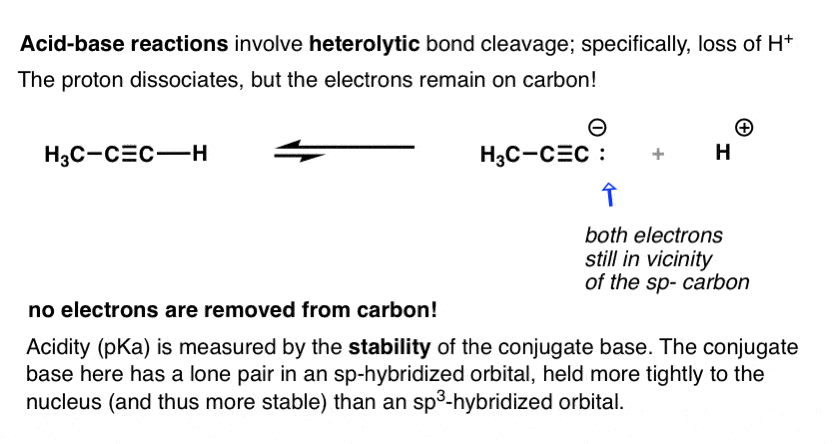
Orbital diagram of carbon before sp3 hybridization
8.2 Hybrid Atomic Orbitals - Chemistry sp 2 Hybridization. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry (). Hybridisation - Mixing Up Orbitals with sp, sp2, sp3 - Biochem.co Carbon - Hybridisation forms sp3 orbital. This leaves 4 valence electrons which will each overlap with the s orbital of a Hydrogen to form a σ (sigma) bond. These hydrogens space themselves as far apart as possible, leading to the tetrahedral structure of methane. 3D animation of methane. Produced on ChemSketch. Solved Consider the electron configuration. Write the - Chegg Write the orbital diagram of carbon before sp3 hybridization. ... Write the orbital diagram of carbon before sp3 hybridization. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
Orbital diagram of carbon before sp3 hybridization. Hybridization of Atomic Orbitals The molecular, sp 3 orbitals are arranged in a tetrahedron, with bond angles of 109.5 o. Each of the 1s orbitals of H will overlap with one of these hybrid orbitals to give the predicted tetrahedral geometry and shape of methane, CH 4. Hybridization also changes the energy levels of the orbitals. The 2s orbital of carbon is lower in energy than the 2p orbitals, since it is more penetrating. What does the atomic orbital diagram of carbon look like before sp^3 ... with sp3 hybridized orbitals of 25% s character and 75% p character. SIDENOTE: Because the 2p orbital constitutes most of the sp3 orbitals ( 75% ), and because the 2p orbitals were higher in energy than the 2s orbitals, the sp3 orbitals are closer in energy to the original 2p orbitals than to the original 2s orbital. Answer link Solved Write the orbital diagram of carbon before sp | Chegg.com Question: Write the orbital diagram of carbon before sp hybridization. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (6 ratings) sp3 Hybridization | Introduction to Chemistry | | Course Hero In the ammonia molecule (NH 3 ), 2s and 2p orbitals create four sp 3 hybrid orbitals, one of which is occupied by a lone pair of electrons. In a water molecule, two sp 3 hybrid orbitals are occupied by the two lone pairs on the oxygen atom, while the other two bond with hydrogen. Term tetravalenthaving a valence of 4
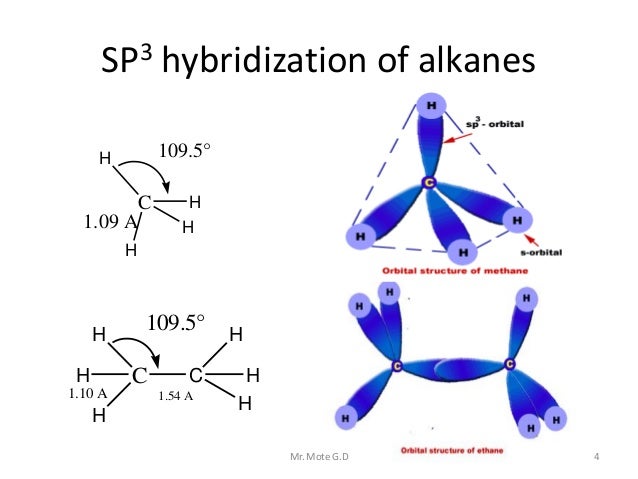
Answered: Write orbital diagrams (boxes with… | bartleby Solution for Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. close. Start your trial now! First week only $4.99! arrow_forward. learn. write. tutor. study resourcesexpand_more. Study Resources. We've got the study and writing resources you need for your ... Hybridization of Carbon - Molecular Geometry and Bond Angles Example: Hybridization of graphite. 3. sp 3 Hybridization. When the carbon atom is bonded to four other atoms the hybridization is said to be sp 3 type. Here 1 s orbital and 3 p orbitals in the same shell of an atom combine to form four new equivalent orbitals. The arrangement is tetrahedral with a bond angle of 109.5 o. Example: Hybridization ... Write orbital diagrams (boxes with arrows in them) to ... - Brainly.com answered • expert verified Write orbital diagrams (boxes with arrows in them) to represent the electron configurations of carbon before and after sp hybridization. Advertisement andrewweltz2136 is waiting for your help. Add your answer and earn points. Expert-verified answer meerkat18 Carbon has an electron configuration of 1s^2 2s^2 2p^2. Hybridization - sp, sp2, sp3, sp3d, sp3d2 Hybridized Orbitals ... - BYJUS The new orbitals formed are called sp3 hybrid orbitals. These are directed towards the four corners of a regular tetrahedron and make an angle of 109°28' with one another. The angle between the sp3 hybrid orbitals is 109.28 0 Each sp 3 hybrid orbital has 25% s character and 75% p character. Example of sp 3 hybridization: ethane (C 2 H 6 ), methane.
What is the hybridization of the orbitals? - Vivu.tv hybrid orbital - A hybrid orbital is an orbital formed by the combination of two or more atomic orbitals. Example: The orbitals that form around beryllium in BeF2 are a combination of s and p orbitals called sp hybrid orbitals.. Hybrid orbitals orbitals used to describe bonding that are obtained by taking combinations of atomic orbitals of ... Sp3, Sp2 and Sp Hybridization, Geometry and Bond Angles Oxygen's 6 valence electrons sit in hybridized sp³ orbitals, giving us 2 paired electrons and 2 free electrons. Since water's oxygen is sp³ hybridized, the electronic geometry still looks like carbon (for example, methane). But the model kit shows just 2 H atoms attached, giving water the Bent Molecular Geometry. Sp² Hybridization Hybridization | Department of Chemistry 1) The bulk of the electron density is directly between the two carbon atoms, indicative of a sigma bond. 2) The shape of the hybrid matches what orbitals were used to make it. For this case, sp 3 hybrids are 3 parts p orbitals and 1 part s orbital. The end result is an orbital that is mostly p shaped but it a little bit lop-sided. four way switching diagram Hub-based networks :: chapter 12. switching case studies :: lan. Write the orbital diagram of carbon before sp3 hybridization — untpikapps. 32a & 63a 3 pole change over switch four way switching diagram. ... nmr signals hybridization orbital sp3 untpikapps. Switching Solutions For Multiple Battery Banks - Blue Sea Systems ...
Hybridization-sp3 Hybridization-sp2 Hybridization-sp Hybridization ... The process of hybridization in which one s-orbital and three p-orbitals overlap to produce four hybrid-orbital is known as Sp 3 -hybridization. These hybrid-orbital are identical in shape and energy. These orbital are known as Sp 3 -hybrid orbitals. Sp 3 -orbitals are at an angle of 109.5 o from each other.
Write the orbital diagram of carbon before sp^3 hybridization. | Study.com Answer to: Write the orbital diagram of carbon before sp^3 hybridization. The element is given as carbon. The atomic number of C is 6 and its configuration...
Hybrid orbitals | Chemical bonds (video) - Khan Academy In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. Created by Jay. Google Classroom Facebook Twitter Email Sort by: Tips & Thanks
Orbital Diagram Of Carbon Before Sp3 Hybridization * The two carbon atoms form a σ sp 3-sp 3 bond with each other due to overlapping of sp 3 hybrid orbitals along the inter-nuclear axis. sp 3 Hybridization. The ground state configuration of carbon is 1s 2 2s 2 2px 1 2py 1. The p orbitals are equal in energy and said to be degenerate.
The orbital diagrams (boxes with arrows) to represent the electron ... The orbital diagrams (boxes with arrows) to represent the electron configuration of carbon before and after sp hybridization. Concept Introduction: Carbon has an electron configuration of 1 s 2 2 s 2 2 p 2 . during hybridization, the s and p orbitals of carbon combine to form two sp hybrid orbitals. Question Chapter 10, Problem 58E
sp Hybridization | Introduction to Chemistry | | Course Hero In sp hybridization, the s orbital overlaps with only one p orbital. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. sp orbitals are oriented at 180 degrees to each other. Terms hybrid orbitalformed by combining multiple atomic orbitals on the same atom
sp³ hybridized orbitals and sigma bonds (video) | Khan Academy A hybridized orbital is a combination of s and p. Hybridized sp3 orbitals are the orbitals when carbon bonds with things like hydrogen or really when it bonds with anything. So if you looked at a molecule of methane, and people talk about sp3 hybridized orbitals, all they're saying is that you have a carbon in the center.
SOLVED:Draw orbital diagrams (boxes with arrows in them) to ... - Numerade VIDEO ANSWER: so before we hybridize carbon, Adam is goingto have the two f formidable and three to Pee or girls. ... Draw orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. Answer. See drawing. View Answer. Related Courses. Chemistry 101. Chemistry Structure and ...
Write orbital diagrams (boxes with arrows in them) to repres - Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Write orbital diagrams (boxes with arrows in them) to represent the electron configurations of carbon before and after sp hybridization..
Solved Consider the electron configuration. Write the - Chegg Write the orbital diagram of carbon before sp3 hybridization. ... Write the orbital diagram of carbon before sp3 hybridization. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
Hybridisation - Mixing Up Orbitals with sp, sp2, sp3 - Biochem.co Carbon - Hybridisation forms sp3 orbital. This leaves 4 valence electrons which will each overlap with the s orbital of a Hydrogen to form a σ (sigma) bond. These hydrogens space themselves as far apart as possible, leading to the tetrahedral structure of methane. 3D animation of methane. Produced on ChemSketch.
8.2 Hybrid Atomic Orbitals - Chemistry sp 2 Hybridization. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry ().


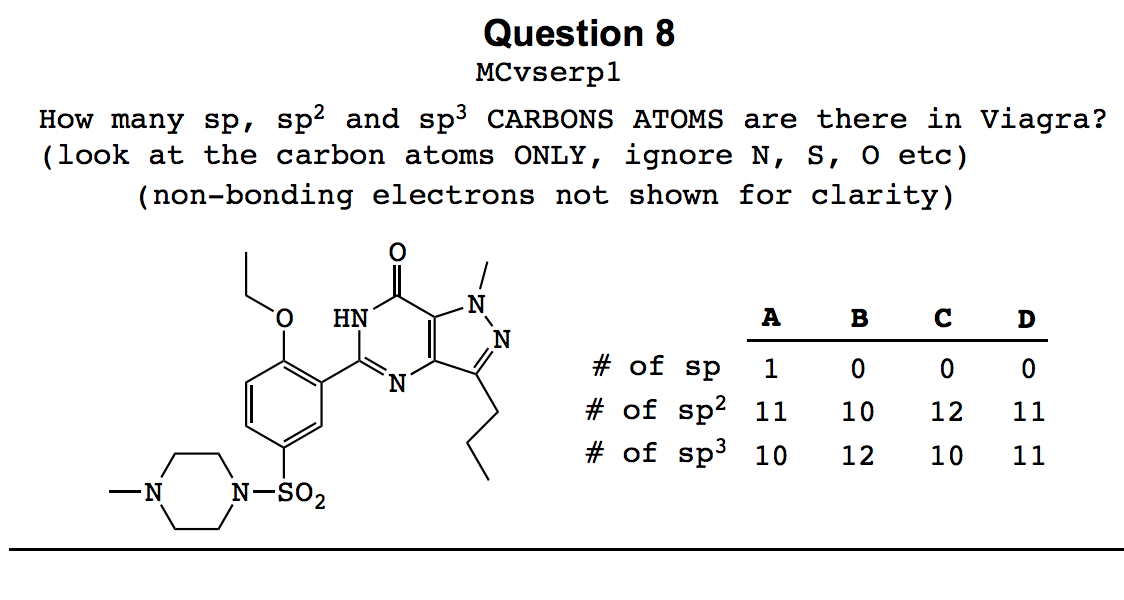



0 Response to "40 orbital diagram of carbon before sp3 hybridization"
Post a Comment