39 label the phase diagram of pure solvent and a solution
Label the phase diagram of pure solvent and a solution Freezing point of solution GAS Solution Pure solvent 1 atm Boiling pointFreezing point of of solvent solvent ??? 11 SOLID AT AP Boiling pointLIQUID of solution Temperature.
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Label the phase diagram of pure solvent and a solution. Shown above is a phase diagram for water. Well look now at the phase diagram for sodium chloride solution in some detail. Phase diagrams of pure water and an aqueous solution of a nonvolatile solute. Pure a and pure b are also considered to be α and β phases respectively.
Label the phase diagram of pure solvent and a solution
Chemistry. Chemistry questions and answers. Label the phase diagram of pure solvent and a solution. Freezing point of solution ΔΤ, Solution Pure solvent 1 atm Boiling point of solution SOLID LIQUID AP Pressure AT) GAS Boiling point of solvent Freezing point of solvent Temperature.
Suppose you have a pure substance at three different sets of conditions of temperature and pressure corresponding to 1, 2 and 3 in the next diagram. Under the set of conditions at 1 in the diagram, the substance would be a solid because it falls into that area of the phase diagram. At 2, it would be a liquid; and at 3, it would be a vapor (a gas).
Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist ...
Label the phase diagram of pure solvent and a solution.
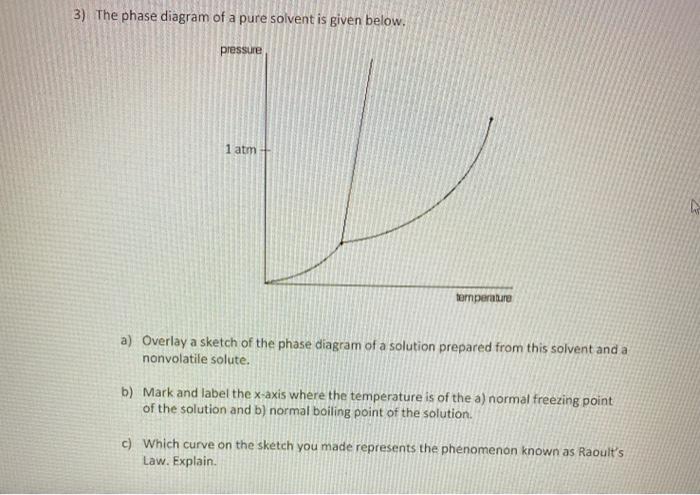
Transcribed image text: The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fp_solv) and boiling (bp_solv) points for the pure solvent and the normal freezing (fp_soln) and boiling (bp_soln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent.
Chemistry questions and answers. The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fpsoly) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpsoln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that ...
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. This is …. View the full answer. Transcribed image text: Label the phase diagram of pure solvent and a solution.
The following diagram shows a close up view of the vapor pressure curves for a pure solvent and a solution containing a nonvolatile solute dissolved in this solvent. Which curve is the pure solvent and what happens to the vapor pressure when the solute is is dissolved in the solvent?


0 Response to "39 label the phase diagram of pure solvent and a solution"
Post a Comment