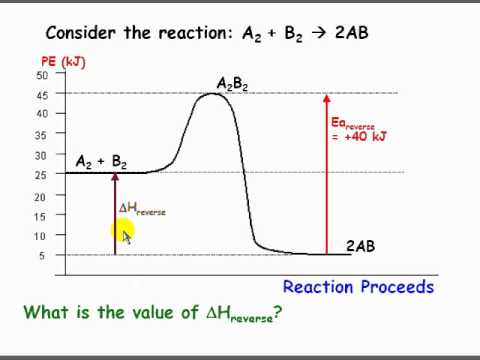
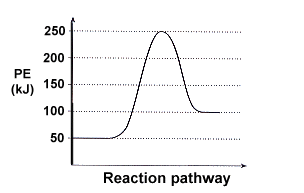
36 what is the enthalpy of reaction from the following energy diagram?
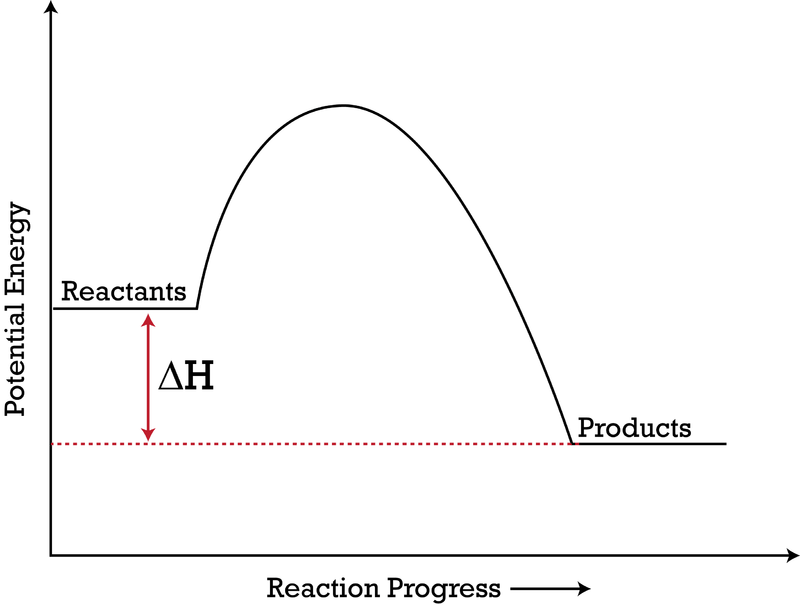
Jul 9, 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
Based on the free energy change, determine whether the reaction ... What is the sign of the enthalpy change for an endothermic reaction? exothermic?4 pages
Jan 9, 2020 — Click here to get an answer to your question ✍️ What is the enthalpy of reaction from the following energy diagram? a. -20kj b.2 answers · 7 votes: A. -20kjExplanation:The reaction is exothermic therefore enthalpy = 20 - 40 = -20

What is the enthalpy of reaction from the following energy diagram?
Hello! I am currently taking a general chemistry course online and it has been a real struggle. The lecture materials provided by the instructor moreso show you how set up a few equations, conversions, or ratios, but they don't really explain in detail what is actually happening during the reaction or why. This is the part that helps me to understand and remember what equations/ratios/conversion methods to use to solve the math part of the problem. I'm just not getting a good understanding from ...
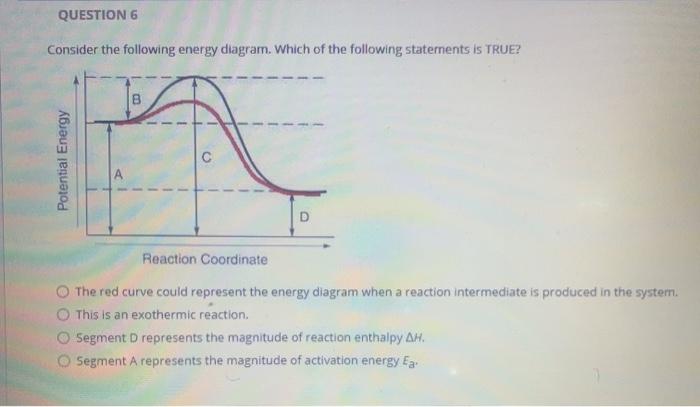
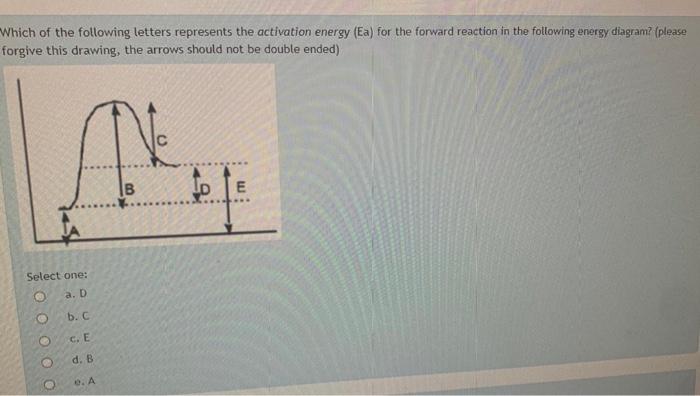
What letter represents the potential energy of the activated complex? 8. Is the reverse reaction endothermic or exothermic? endothermic. 9. If a catalyst were ...8 pages
Apr 9, 2018 — The enthalpy of a system, H , measures the sum of its internal and potential energy. The reaction here see a decrease in chemical potential ...2 answers · Since heat is released for C3H8(g)+5O2(g)→3CO2(g)+4H2O(g)+2219.9 kJ, we say that ΔH∘C=−2219 ...
What is the enthalpy of reaction from the following energy diagram?.
**Firstly, I would like to thank the community and their posts that were really helpful.** **This is a pretty long post, but will have detailed path that I followed to study for the exam.** Preparation- Time is not an issue here, you can study in two weeks or even two months, depends on personal grasping and touch with the subject. (My suggestion: Two weeks time, (even if you are starting from scratch) is more than enough as long as you are willing to patiently invest at least 8 hours a day.)...
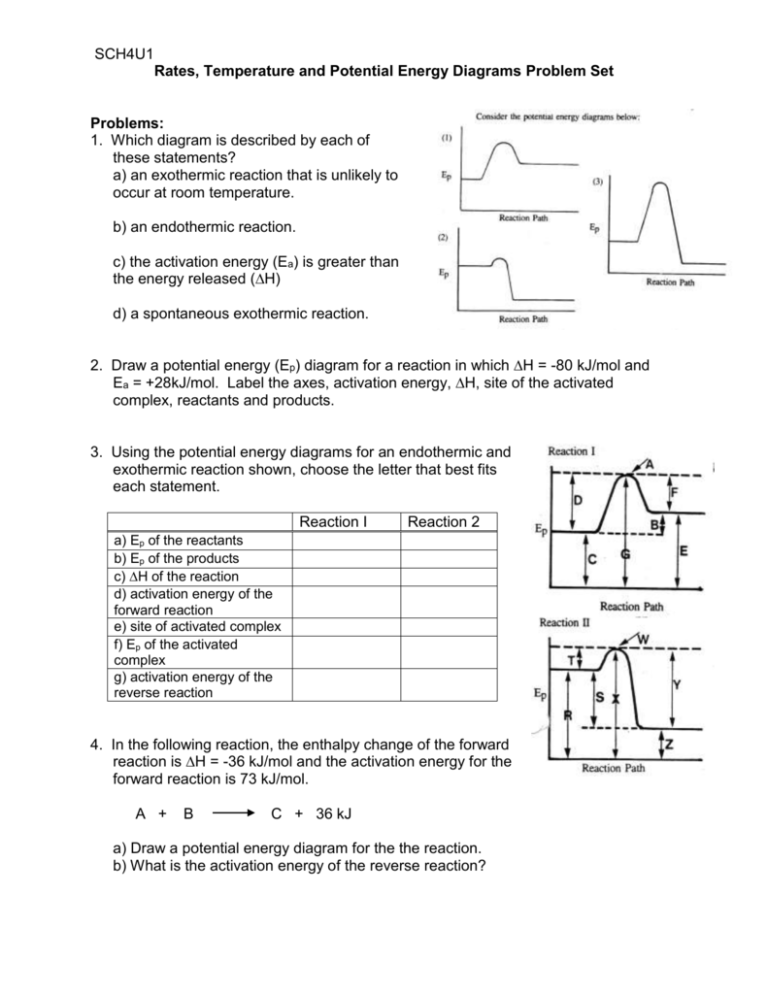
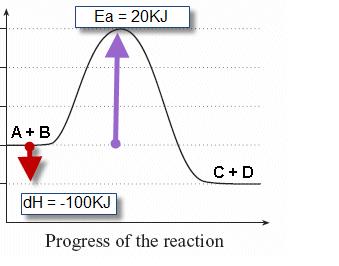
Click here to get an answer to your question ✍️ Given the following diagram for the reaction A + B → C + D . The enthalpy change and activation energy ...1 answer · Top answer: The enthalpy change for the backward reaction is equal in magnitude and opposite in sign to that for the forward reaction.Thus the magnitude of the ...
Headache, woke up later than usual. Only Chem P1 tips today. To those who are currently taking their papers, good luck with them. ​ Second last day, time sure fly fast. Wash up, have a good breakfast, drink some water, whatever it takes to not burn out. Go run an hour before the exam if you wish to, just don't be late. If you forgot your calculator, either borrow from others or learn quick maths. ​ Papers today: \- (1153/1151/1152)/01 Chinese/Malay/Tamil B P1 08:00 - ...
Oct 20, 2021 — From the given energy diagram we conclude that this is an exothermic reaction diagram. Delta H=H_{Reactant}-H_{Product}. Delta H=40kJ-20kJ.
\[[https://pubchem.ncbi.nlm.nih.gov/periodic-table/png/Periodic\_Table\_of\_Elements\_w\_Chemical\_Group\_Block\_PubChem.png](https://pubchem.ncbi.nlm.nih.gov/periodic-table/png/Periodic_Table_of_Elements_w_Chemical_Group_Block_PubChem.png) \] or \[[https://ptable.com/#Properties](https://ptable.com/#Properties) \] In the last post, I mentioned the concept of activation barriers: reactions require an energy input to proceed from starting materials to a transition state even if there is a net re...











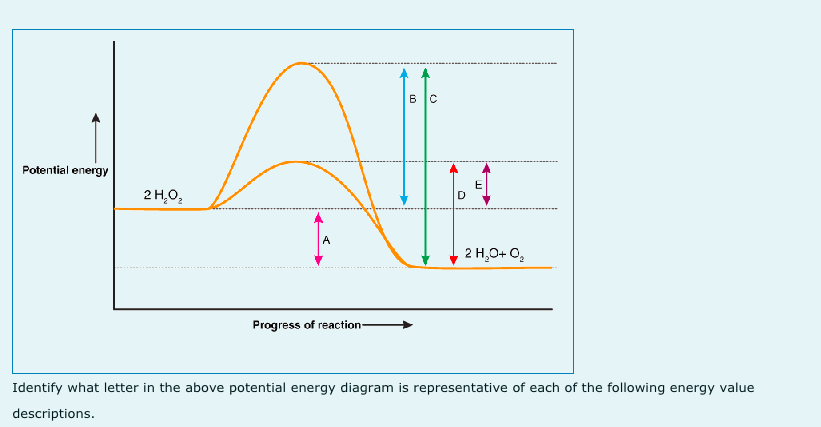
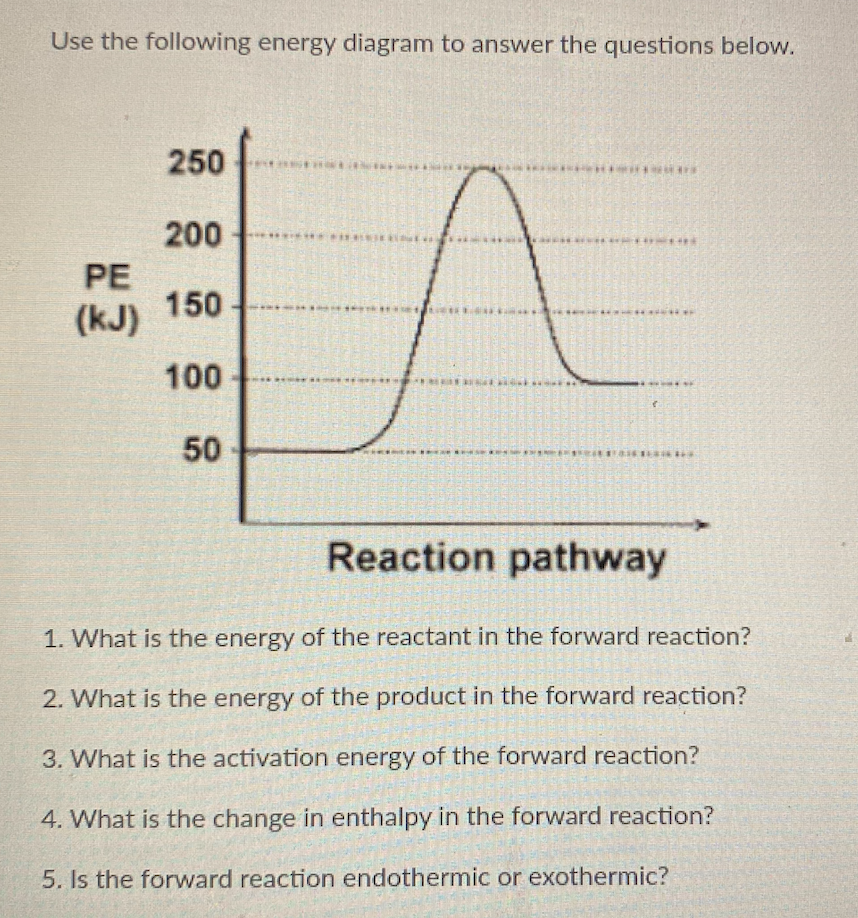

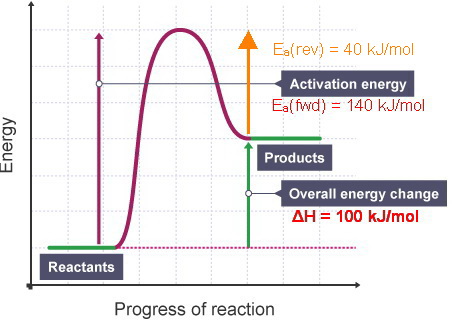
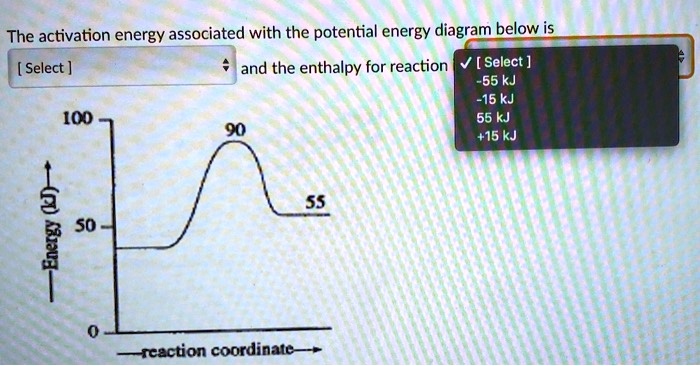






0 Response to "36 what is the enthalpy of reaction from the following energy diagram?"
Post a Comment