34 molecular orbital diagram for n2
Molecular orbital diagram for b2. By drawing molecular orbital diagrams for b2 c2 n2 o2 and f2 predict which of these homonuclear diatomic molecules are magnetic. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.
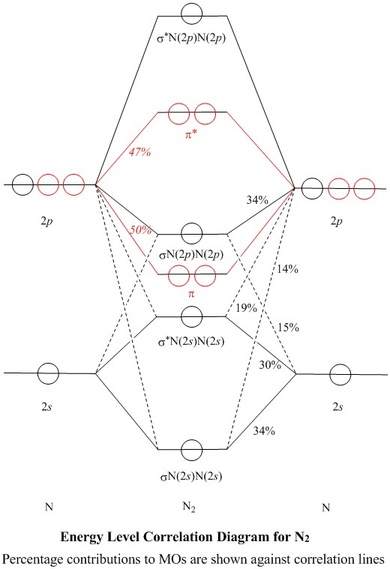
OM diagram for the N2 molecule. Source: Gabriel Bolívar. Note that this diagram is exactly the same as for the C 2 2- anion . This means that N 2 and C 2 2- are isoelectronic. However, this fact does not imply that both species behave in the same way.
Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means.
Molecular orbital diagram for n2
Draw the molecular orbital diagram of N2. Also find its bond order and magnetic · Asked by Topperlearning User | 13th Jun, 2016, 02:45: PM. Expert Answer: ...
A draw the molecular orbital diagram. N 2 has a bond order of 3 and is diamagnetic. Bonding order is 2 and it is diamagnetic. Interact and form molecular orbital s. B calculate the bond order. Molecular orbital diagram for n2 o2 c2 f2 also h2o. Consider the h 2 molecule for example. Sp mixing causes the σ g and σ u mos to be pushed apart in energy.
20/03/2019 · Energy level diagram for Molecular orbitals. ... If N b = Na,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular …
Molecular orbital diagram for n2.
The molecular orbital diagram for ClO - is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. the atom stlcc edu, worksheet energy levels sublevels orbitals name of, molecular orbital theory purdue university, electron filling sequence article about electron filling, 6 4 electronic structure of atoms electron configurations, constructing the o2 molecular orbital ...
How many molecular orbitals will have an N value of 2? Explanation: The atomic orbitals with an n value of 2 are the 2s orbital and the three 2p orbitals.
2 answersLet me explain the molecular orbital diagram of N2 using its diagram. · one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons · so first 2 ...
What is the MO diagram and bond order for N2 ( in Urdu / Hindi) Nitrogen (N 2) molecule: Nitrogen atom has electronic configuration 1s2, 2s2, 2p3. Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px.
How to Make the Molecular Orbital Diagram for B2- (Bond Order, Paramagnetic or Diamagnetic). Principia. Principia. 1 answerThe MO diagram of the NF molecule can be drawn as below. The energies of the atomic orbital of N and F are different. The atomic orbital s of F are.... 1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in ...
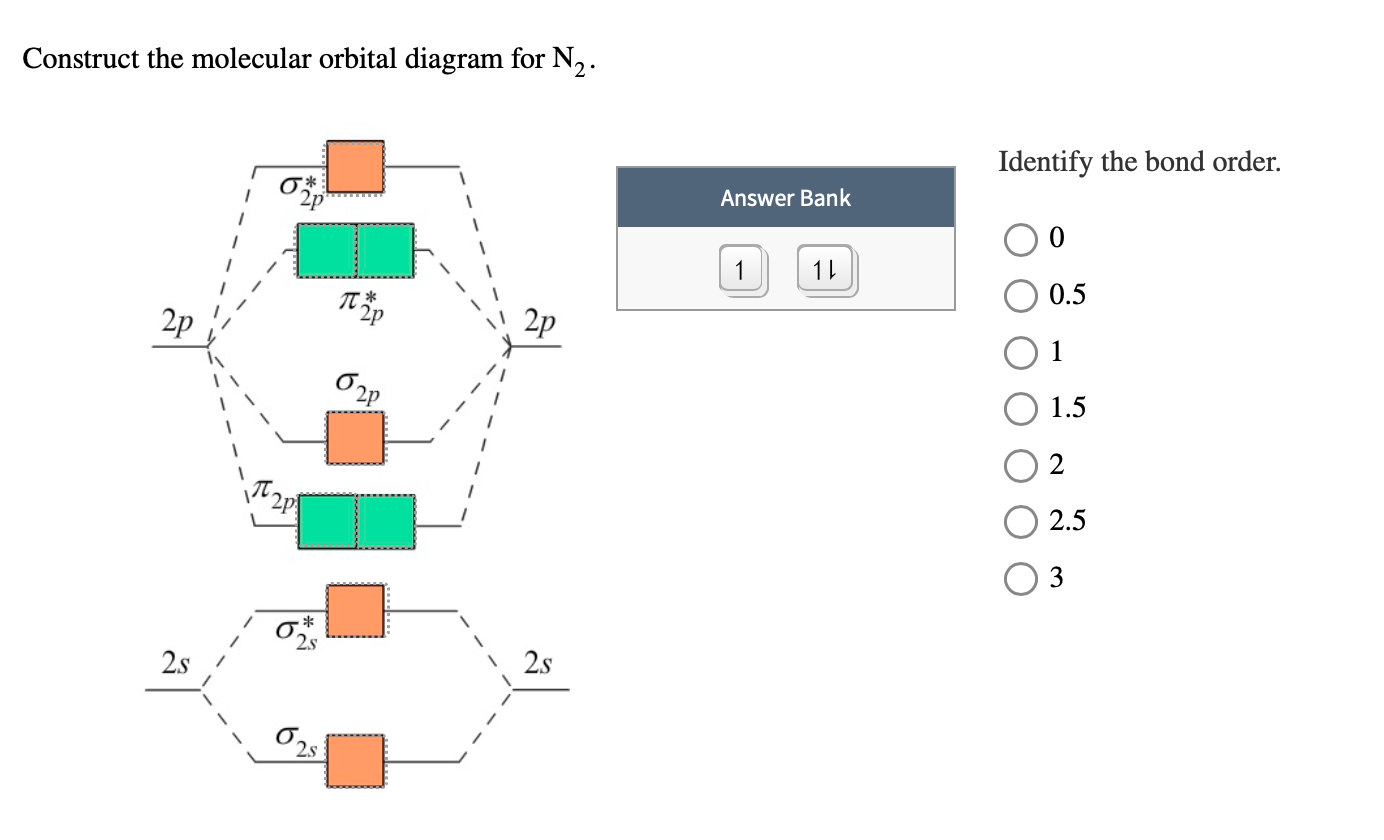
Molecular orbital diagram of dinitrogen molecule, N 2. There are five bonding orbitals and two antibonding orbitals (marked with an asterisk; orbitals involving the inner 1s electrons not shown), giving a total bond order of three. Atomic nitrogen, also known as active nitrogen, is highly reactive, being a triradical with three unpaired electrons.
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
ANSWERS TO MOLECULAR ORBITALS PROBLEM SET. 1. (a). N2. +(13 e-): σ2. 1sσ*2. 1sσ2. 2sσ*2. 2sπ2. 2pπ2. 2pσ1. 2p. N2. 2+(12 e-): σ2.1 page
Molecular Orbital Diagram Of N2 Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule.
Tagged as: #digitalkemistry, Bond order of Nitrogen molecule, chemical bonding, chemical bonding and shapes of molecules, chemistry, class 11 chemistry molecular orbital theory, class 11 chemistry molecular orbital theory notes, class 11 chemistry notes, digital kemistry, How do you draw a molecular orbital diagram, How do you find the bond ...
21/11/2018 · We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The ...
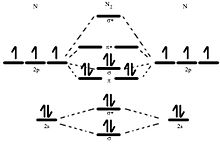
Click here to get an answer to your question ✍️ Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, ...1 answer · Top answer: Formation of N2 molecule: Electronic Configuration, σ 1s^2<σ *1s^2<σ 2s^2<σ *2s^2<[pi 2px^2 = pi 2px^2]<<σ 2pz^2 Bond order = (Nb - Na)/2 = (10 - ...
Answer (1 of 3): In O2 2+, there is 14 electrons. So, it's MOT is comparable to N[code ]2[/code] & the MOT diagram will look like this :
N2 is a very stable 10-valence-electron molecule, isoelectronic with CO and with [CN] · The formal bond order of N2 is 3, from about one σ-bond and two π-bonds ...
14+ Energy Level Diagram Of N2.As bond dissociation energies are directly proportional to the bond order, therefore, the dissociation energies of these molecular species are in the order diagram for o2+ is wrong because 2p atomic orbital of 2nd o atom will have only 3 e Energy level diagram part 1.
When we make the molecular orbital energy level diagram of f2 molecule then, we will get this configuration: 1σs 2, 1σ*s 2, 2σs 2, 2σ* 2, σ2pz 2, π2p x 2, π2p y 2, πp x * 2, π2p y * 2. From this electronic configuration, we can see that there are a total of ten bonding molecular orbitals and eight antibonding molecular orbitals.
1 Answer. 1. Electronic configuration of N atom is 1s2 2s2 2p3. 2. Electronic configuration of O atom is 1s2 2s2 2p4. 3. Electronic configuration of NO molecule is σ1s2 σ*1s2 σ2s2 σ*2s2 π2px2 π2py2 π2pz2 π*2px1. 4. Bond order = N b−N a 2 N b − N a 2 = 10−5 2 10 − 5 2 = 2.5.
According to our diagram, there are 8 bonding electrons and 6 antibonding electrons, providing a bond order that (8 − 6) ÷ 2 = 1. Thus F2 is guess to have a secure F-F single bond, in commitment with experimental data. Example (PageIndex3): Diatomic Sulfur. Use a qualitative molecular orbital energy-level diagram to predict the electron ...
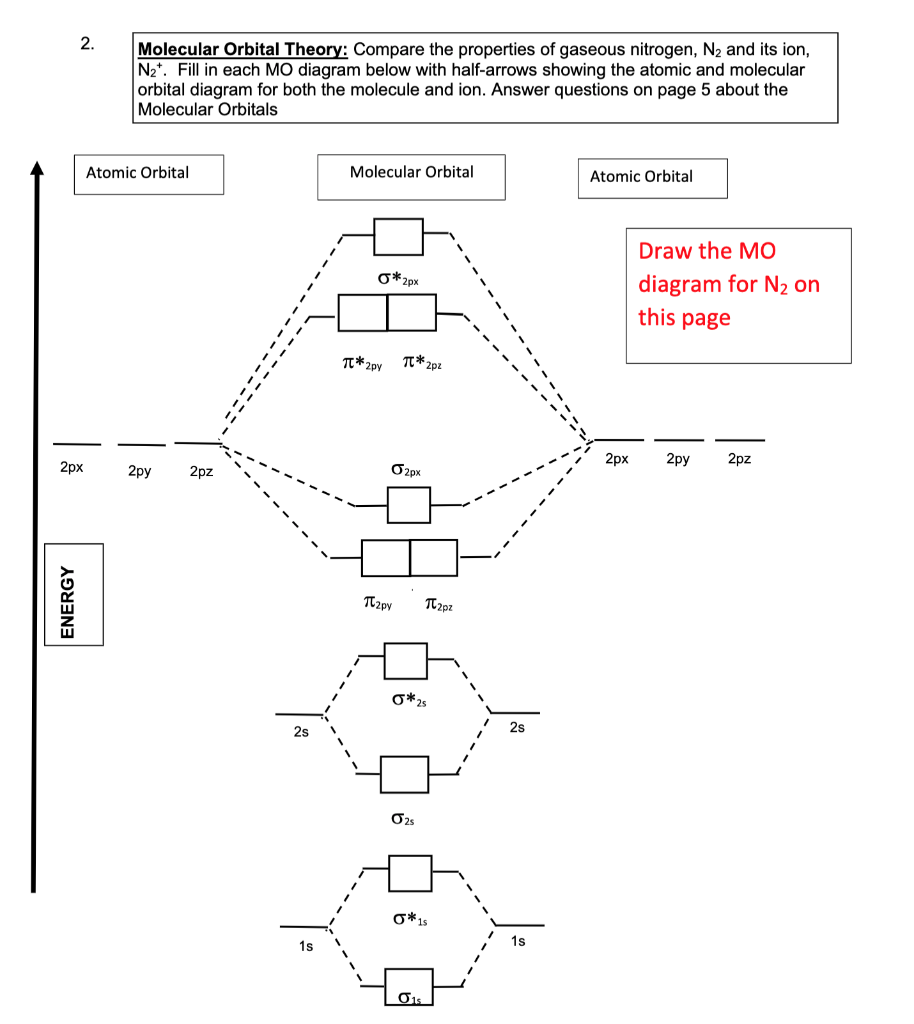
Molecular Orbital Diagram for Nitrogen Gas ( 1 ion) (N2( )). Fill from the bottom up, with 9 valence electrons total. Comparing and Contrasting the Molecular Orbital Diagram s of N2- and N2+.In this video lecture Molecular Orbital Energy Level Diagram s for N2, N2 , N2-, N2 2- C2 and B2 are.... Fig. No. 1 Molecular Orbital Diagram for H2 molecule. Comparison of N2 and N2+ ion N 2 + ion is for ...
For more detailed knowledge you can refer to the polarity of N2. Molecular Orbital Diagram of N2 Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule.
The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n (2px) 2 n (2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons. Molecular orbital diagram for c2 2-. The bond order of B2, C2, and N2 are 1, 2, and 3, respectively.
N2 (σ2s)2(σ∗2s)2(π2py,π2pz)4(σ2px)2: 3: O2 ... So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, ...
Answer: Answer: N2 < N2 ^- < N2 ^2- Because, bond length is inversely proportional to bond order. Bond order of N2 is 3.0, of N2^- is 2.5 and of N2^2- is 2.0. Bond orders can be obtained from their molecular orbital diagrams. In N2^- and N2^2-, the additional electron (s) go (es) to antibondin...
To obtain the molecular orbital energy-level diagram for \(\ce{O2}\), we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure 9.10.1 . We again fill the orbitals according to Hund's rules and the Pauli principle, beginning with the orbital that is lowest in energy.
Solved Using The Molecular Orbital Diagram Depicted Below Which Species Have Bond Order Of 3 2p 2p 02p 72p Energy 2s Oa B2 B 02 2 C C22 D N2 Oeco Of Cn G Molecular orbital diagram for b2 . This interaction introduces an element of s p mixing or hybridization into the molecular orbital theory.
Molecular orbital diagram practice worksheet. For the following elements. the four orbitals as bonding, non-bonding or antibonding. 1 and inserting your answers in the answer blanks. Nitrogen is the seventh element with a total of 7 electrons. 30 M solution of NaCl (58. 69 m 50. The HR Diagram. Molecular Orbital Worksheet 1. 54 m C) 17.
Clearly, Cyanide (CN) lies in a hetero-nuclear diatomic molecular orbital as it contains two different atoms. Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1.
In the Lewis structure of the N2 molecule, over there is a formation of a triple covalent bond stood for by three lines between two atom of Nitrogen. The leftover 2 2p orbitals become two π bonds and electrons making a pair in between the nitrogen atoms will make a sigma bond. VSEPR version assumes that molecular geometry minimizes the ...
d) n2 < n2 + = n2 - < n2 2- 53.On the basis of molecular orbital theory, select the most appropriate option. a) The bond order of O2 is 2.5 and it is paramagnetic
N2O Molecular Orbital Diagram. Molecular orbital diagrams say about the mixing of orbitals in a compound. Using a MO diagram, the bond order of a compound can be determined which gives us an idea about bond length, bond stability as well. Nitrous oxide's MO can be drawn easily by understanding the basics. So for that let's have a look at ...
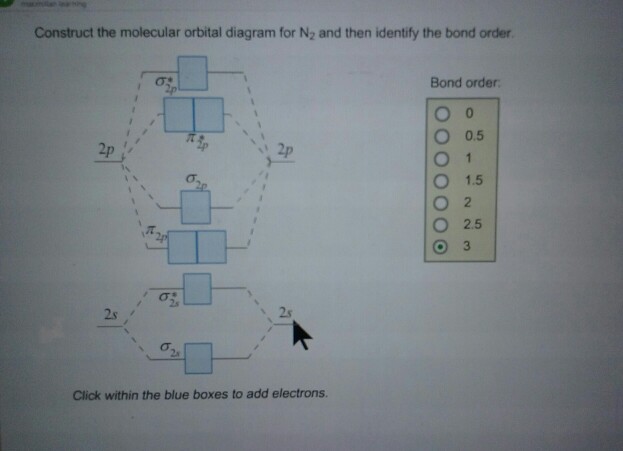
How to draw the molecular orbital diagram of N2? Expert Answer: Molecular orbital diagram of N2 BO = [Nb-Na] = [10-4] = 3 Since all the electrons in nitrogen are paired, it is diamagnetic molecule.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine …
Molecular orbital Diagram that N2 Molecular orbitals exist in molecules where each molecule has its electron construction in regards to a sigma bond and also pi bond. According come molecular orbital theory, that tells about magnetic nature, security order, and also the number of bonds in a molecule.
17/10/2018 · Molecular orbital (MO) diagram for N2 and N2^- $-$\mathrm{p}$ interaction moving from $\ce{Li2}$ to $\ce{F2}$. The $\mathrm{s}$-$\mathrm{p}$ interaction is the bonding interaction between the $\mathrm{2s}$ orbital of one atom and the $\mathrm{2p_{z}}$ orbital of another atom which (among other things) increases the energy of the $\mathrm.Molecular …
The MO method for N2+ gives the bond order equal to 2.5. But first, we look at the diagram of molecular orbitals for N2 (the bond order for the nitrogen molecule is 3). the N2+ molecule). That is, the bond order for N2+ is 2.5.
Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s (2),sigma2s* (2),pi2p (4),sigma2p (2). N2 2 Molecular Orbital Diagram — UNTPIKAPPS from www.untpikapps.com
Molecular orbital diagram of a complex including an oxido ligand. 24. Molecular orbital (MO) diagram for N2 and N2^-2. Orbital mixing in square planar D4h complexes. 2. Energy level of hybrid orbitals in the molecular orbital energy diagram. 8. Shape of nickel tetracarbonyl. 6.
Q. Place the species B2+, B2, and B2- in order of increasing bond length and increasing bond energy. Solved • Nov 27, 2018. MO Theory: Homonuclear Diatomic Molecules. Q. The highest occupied molecular orbital of a molecule is abbreviated as the HOMO. The lowest unoccupied molecular orbital in a molecule is called th...
















![Best Answer] draw the molecular orbital diagram of N2 and ...](https://hi-static.z-dn.net/files/d20/b492acf8cb9ff01954c3929a3b7a93c7.jpg)








0 Response to "34 molecular orbital diagram for n2"
Post a Comment