37 in an electron dot diagram, the symbol for an element is used to represent
30 Mar 2018 — In an electron dot diagram, the symbol for an element is used to represent the nucleus. For example : As we know that arsenic has '5' valence ...2 answers · 10 votes: A the nucleus bruh it can't be anything else
When we draw the Lewis Structure for the Carbon you'll put four "dots" or valance electrons around the element symbol (C). What is the maximum number of dots in a Lewis dot structure? The maximum number of valence electron dots in the Lewis electron dot diagram is \begin{align*}8\end{align*}.
In an electron dot diagram, the symbol for an element is used to represent the nucleus. For example : As we know that arsenic has '5' valence electrons. So, the symbol (As) is used to represent the nucleus and valance electrons around the is represented by the 'dot'. The Lewis-dot structure of As (arsenic) is shown below. Thanks Useless

In an electron dot diagram, the symbol for an element is used to represent
In a Lews dot structure, the elemental symbol is used to represent the molecule's nucleus. The electrons are then represented by the dots.
Correct answers: 3 question: In an electron dot diagram, the symbol for an element is used to represent
In a electron-dot symbol of an element, the dots are used to represent _____. the valence electrons. How many valence electrons are in the electron-dot symbols for the elements in group 3A (13)? 3. The number of dots in the element dot symbol of nitrogen is _____. five.
In an electron dot diagram, the symbol for an element is used to represent.
16 answersElectron dot diagram is a method of writing the chemical symbol of an element by surrounding it with dots to indicate the number of valence electrons.
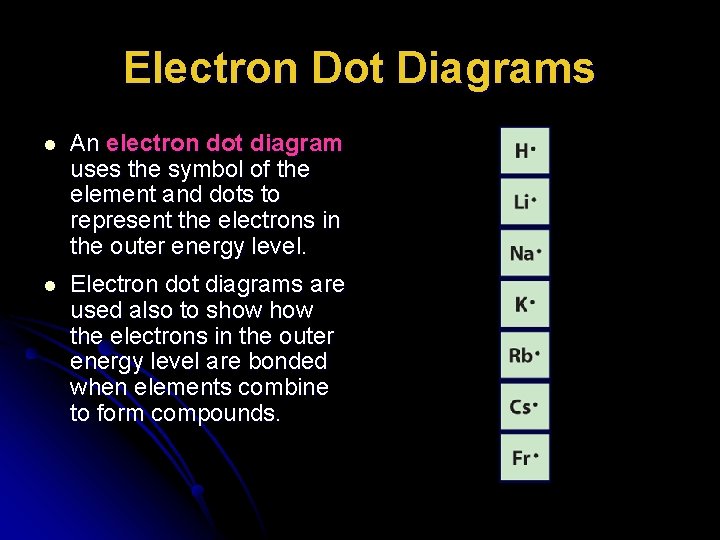
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below.Electron dot diagrams would be the same for each element in the representative element groups.
the nucleus and all non-valence electrons. in an electron dot diagram, the symbol for an element is used to represent what?
The dots simply represent electrons. A single dot represents the one electron. A dash typically represents 2 electrons, and typically represents a covalent bond, i.e. shared electron density between two positively charged atomic nuclei. Most of the time we represent the valence electrons only. The inner shell electrons are along for the ride and do not participate in bonding.
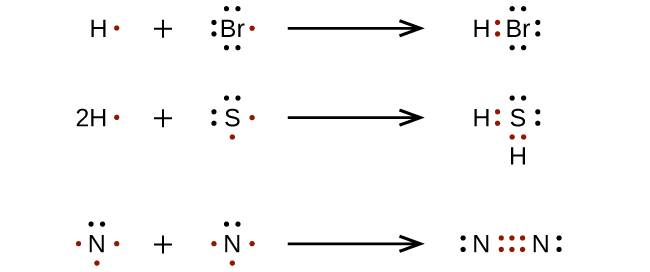
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
In an electron dot diagram, the symbol for an element is used to represent the nucleus and all nonvalance electrons. Log in for more information. Added 9/6/2015 6:48:53 PM. This answer has been confirmed as correct and helpful.
Tell students that one popular method of representing atoms is through Lewis dot diagrams. In a dot diagram, only the symbol for the element and the electrons in its outermost energy level (valence electrons) are shown. Note: In the energy level diagrams students have been using, the electrons are spread out evenly in each energy level. Some ...
In an electron dot diagram, the symbol for an element is used to represent. Electron dot diagrams are diagrams in which the valence electron s of an a to m are shown as dot s d is tributed around the element 's symbol. A beryllium a to m, with two valence electron s, would have the electron dot diagram below.
In an electron-dot symbol of an element, the dots are used to represent _____. a. all of the electrons in the atom b. the valence electrons
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below.
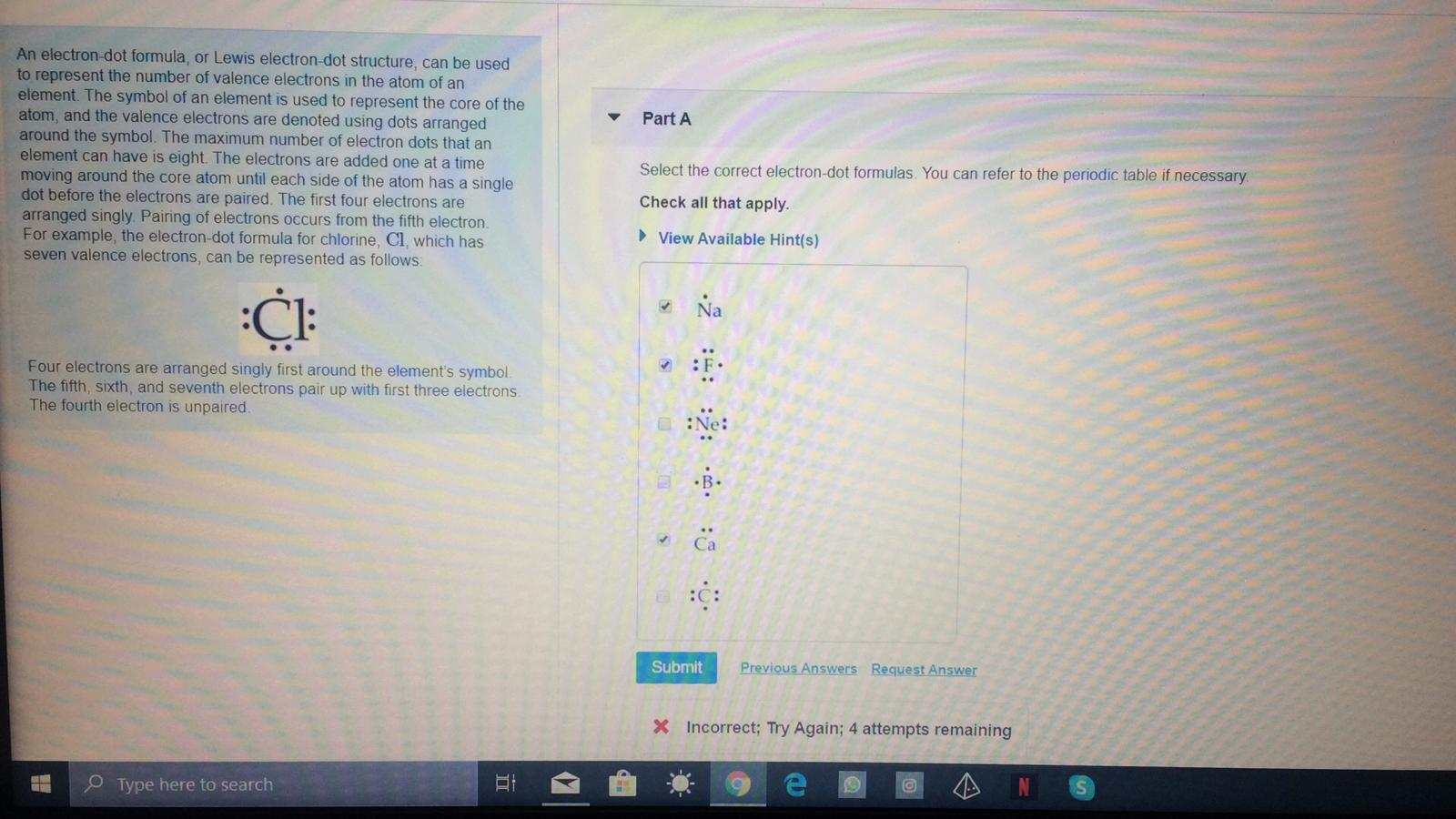
In an electron dot diagram, the symbol for an element is used to represent A. the nucleus. B. the nucleus and all electrons. C. the nucleus and valence ...
What does an electron dot diagram include? Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Electron dot diagrams would be the same for each element in the ...
each dot in an electron-dot diagram represents valence electrons or "available" electrons of each element. For example, Oxygen has 6 valence electrons so it has six dots around it. The number of ...
23 Sept 2021 — Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis dot symbols can be used to predict the number ...
In an electron dot diagram, the symbol for an element is used to represent A. the nucleus. B. the nucleus and all electrons. C. the nucleus and valence electrons. D. the nucleus and all non-valence electrons.
What does the element's symbol in an electron dot structure represent? Atom's valence electrons. What do the dots in an electron dot structure represent? American chemist G.N. Lewis. Who devised the electron dot structure? Showing how the electrons of an atom are involved in chemical bonding.
The Lewis Electron-Dot Symbols of Elements. Gilbert N Lewis is widely known for his use of simple symbolic representations of elements that show valence electrons as dots. You've seen the Bohr's diagram for the first 18 elements. Sometimes it is more convenient to represent the elements by its Lewis electron dot symbol.
Problem. What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: . The valence electron configuration for selenium is 4s 2 4p 4.In the highest-numbered shell, the n = 4 shell, there are six ...
In the Lewis Dot Structure, the element symbol represents the nucleus and inner electrons, and the surrounding dots represent the valence electrons.1 answer · Top answer: The problem asked what the dots in an electron-dot structure represent.Let's define an electron-dot structure:Lewis Dot Structures or Electron Dot Structures ...
24-11-2021 · Many metals can be drawn into a thin wire withour breaking beacause A. cation are still surrounded by electrons when they shift their psiotion in lattice.Bmetals generally have low melting point.C.when metal is struck with a hammer,the position of the anions do not change
In an electron dot diagram, the symbol for an element is used to represent a. The nucleus b. The nucleus and all electrons C. The nucleus and all valence electrons d. The nucleus and all non-valence electrons. Choose the statement that correctly identifies the most stable of the following elements: lithium, carbon, fluorine, and neon a. Lithium ...
Transcribed image text: QUESTION 5 In an electron-dot symbol of an element, the dots are used to represent the electrons that the element will gain when it forms a compound the electron arrangement all of the electrons in the atom only the electrons that will participate in bond formation the valence electrons
1. Write the symbol for the element. The symbol represents the nucleus and the inner or core electrons for the element. In the image below, a generic symbol, X, is used. There are four sides surrounding the symbol. 2. Determine the number of valence electrons for the element. Use a dot to represent an electron. 3.
BriM19 The symbol in an Electron Dot Diagram is used to represent the nucleus and all the non-valence electrons, The Electron Dot Diagram is also known as the Lewis Dot Symbol. The Lewis Dot Symbol or Electron Dot Diagram, is a symbol of an element with one or more dots representing the valence electrons of an element.
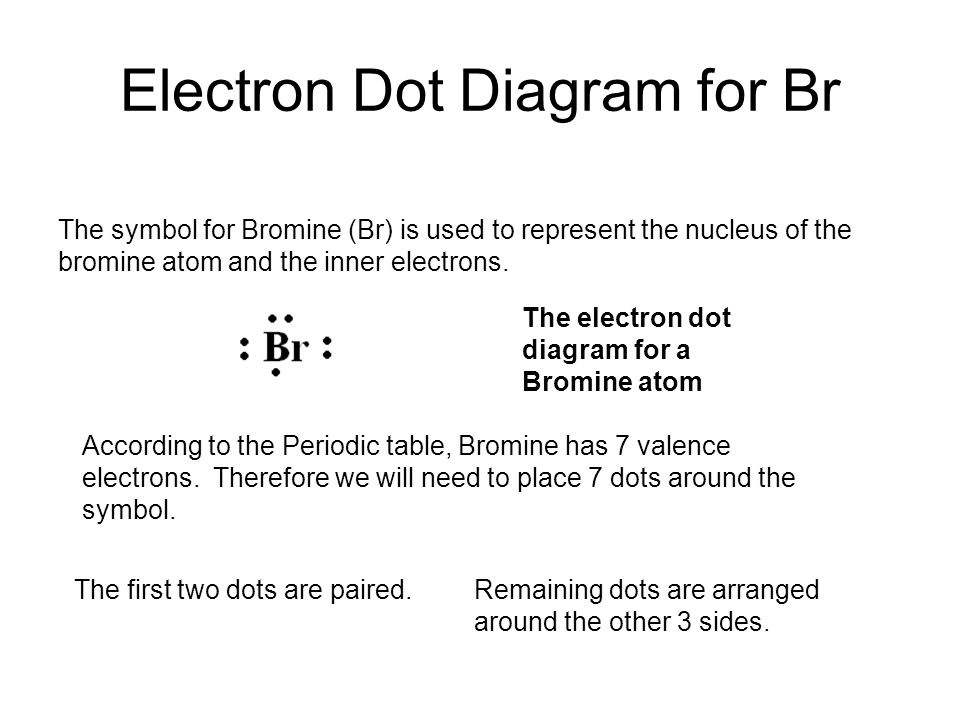
Procedure. 1. Write the symbol for the element. For electron dot diagrams, this symbol represents the nucleus and all of the electrons of the atom except the outermost electrons. The symbol for chlorine is Cl. In an electron dot diagram, this symbol represents the nucleus and the ten electrons in the first two energy levels. 2.
Electron dot formula shows the number of valence electrons for that element with the help of dots. The valence electrons are those electrons that occupy the highest energy level. We can obtain it by using the periodic table. For example, the elements in group IA of the chemical periodic table have 1 valence electron.
What does an electron dot structure show? A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Lewis structure diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion Lewis symbol symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion lone pair two (a pair of) valence electrons that are not used to form a covalent bond octet rule
Lewis symbols are diagrams that show the number of valence electrons of a particular element with dots that represent lone pairs. Lewis symbols do not ...
Electron dot diagrams, which are also called Lewis dot diagrams, are very useful tools in Chemistry. They will give you the ability to determine the type(s) of covalent bonds that an element may make in certain situations. They can also be used to predict the type of ion that an atom might make when it forms an ion.








/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)









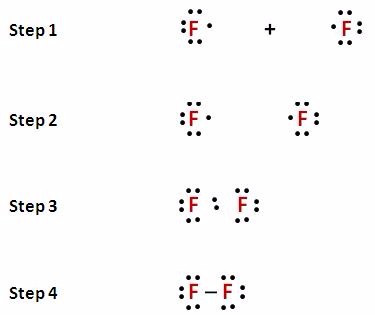




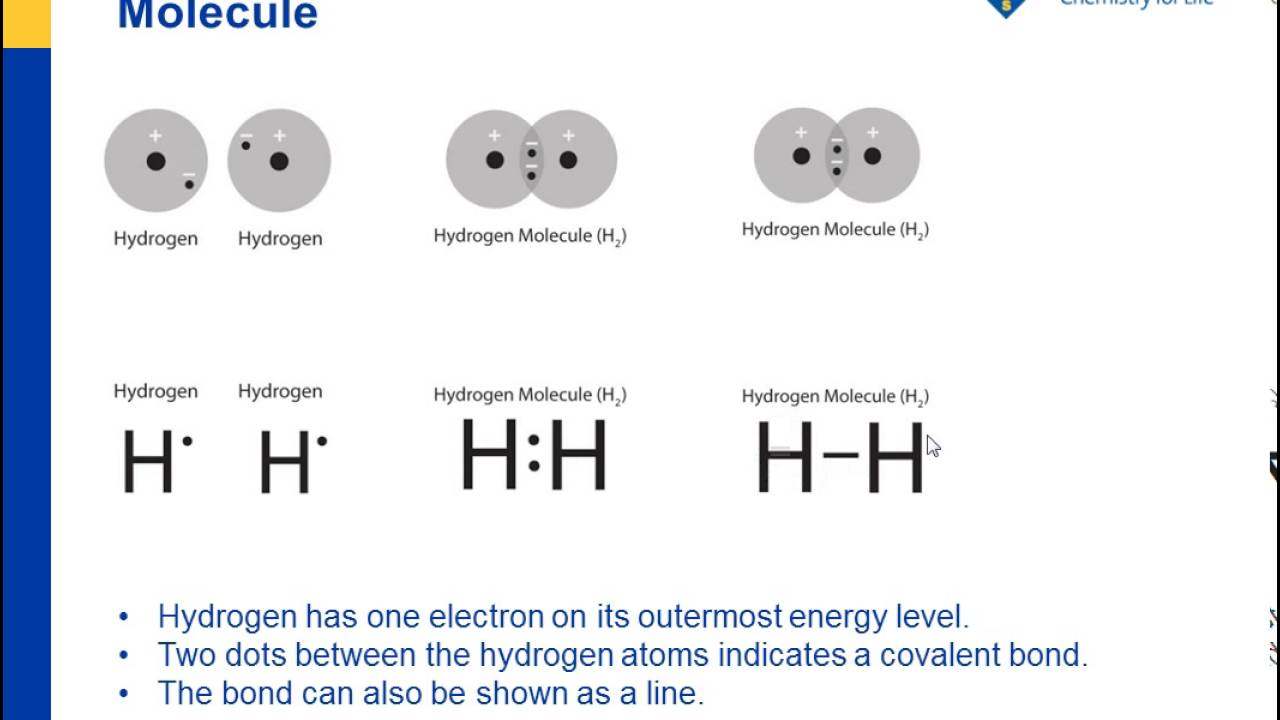
/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)


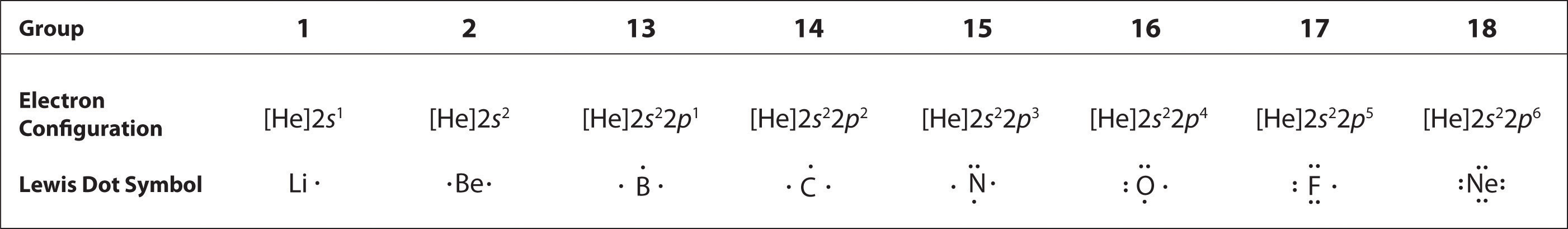
0 Response to "37 in an electron dot diagram, the symbol for an element is used to represent"
Post a Comment