38 nickel cadmium battery diagram
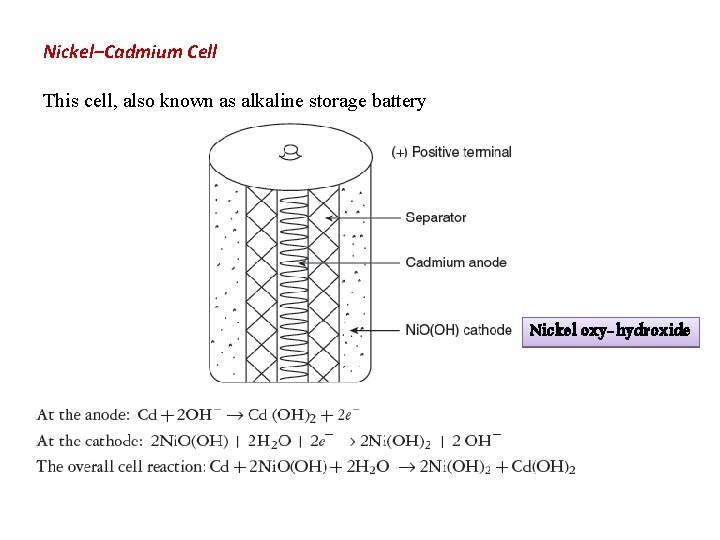
A Nickle-Cadmium battery is superior in most aspects as compared to the lead acid battery. They have Low internal resistance , Can tolerate deep discharge cycle and can also get charged rapidly (usually from 2 hours - 20 minutes) but optimal charging time is about 5 hours. These batteries also have long life time and low maintenance cost. evaluate battery status, give advice on battery status and provide decision support for the operator. Information for the Nickel-Cadmium batteries will be extracted through knowledge engineering utilizing the "Nickel Cadmium Battery experts" at MSFC who are involved with the HST EPS Tes tbed.
All battery connections must be kept tight and coated with grease or other terminal protectant to prevent corrosion. Lead-acid and nickel-cadmium batteries are typically used for electric starting systems. Lead-Acid Lead-acid batteries are readily available, have high output capability, and are relatively inexpensive. Nickel-Cadmium

Nickel cadmium battery diagram
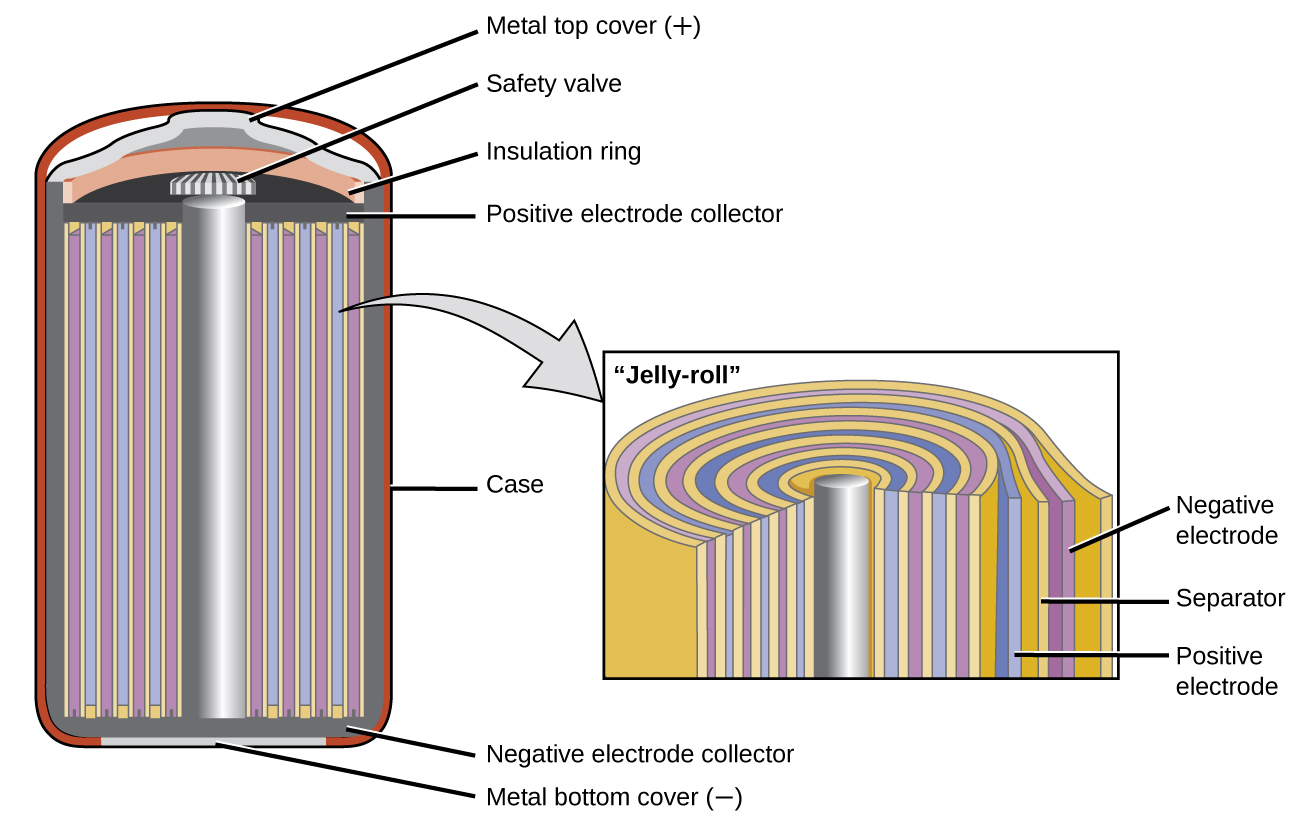
Nickel (hydroxide)–cadmium systems are the most common small rechargeable battery type for portable appliances. The sealed cells are equipped with “jelly roll” ... shorts the cell Nickel cadmium battery diagram, courtesy of Radio Shack. 9 Anatomy of a NiCd cell, cntd •Approximately 10-year-old Sanyo Cadnicacell •Found no apparent metallic formations during optical inspection. 10 Experiment #1: Running an electrochemical cell A nickel-cadmium battery is a system that generates DC voltage by a chemical reaction between the components. In a nickel-cadmium battery, the redox material serves as the nucleus, with a nickel sheet and a separator surrounding it. The voltage of the nickel-cadmium cell is about 1.2 V. As three or four cells are connected in sequence, the output voltage ranges from 3.6 to 4.8 V.
Nickel cadmium battery diagram. vented Ni-Cd cells Batteries are electrochemical devices used to supply energy to electrical and electronic products. Chemical energy stored in a battery is converted into electric current when the battery is discharged. This electric current is produced directly by chemical reactions that occur within the battery. The nickel-cadmium cell is an The cycle lives of Ni-Cd batteries are limited by irreversible structural and physicochemical changes that occur in the porous nickel electrodes as the batteries are cycled between the charged and discharged states. Due to cadmium and nickel concentration levels of about 14% and 24.5% This is a “jelly-roll” design and allows the NiCd cell to deliver much more current ... A diagram is shown of a cross section of a nickel cadmium battery. Nickel-Cadmium Overview Nickel-Cadmium chemistry is similar in some respects to lead-acid in that there are two dissimilar metals in an electrolyte. The basic reaction in a potassium hydroxide (alkaline) electrolyte is: 2 NiOOH + Cd +2 H 2 O Ni(OH) + Cd(OH) However, in NiCd batteries the potassium hydroxide (KOH) does
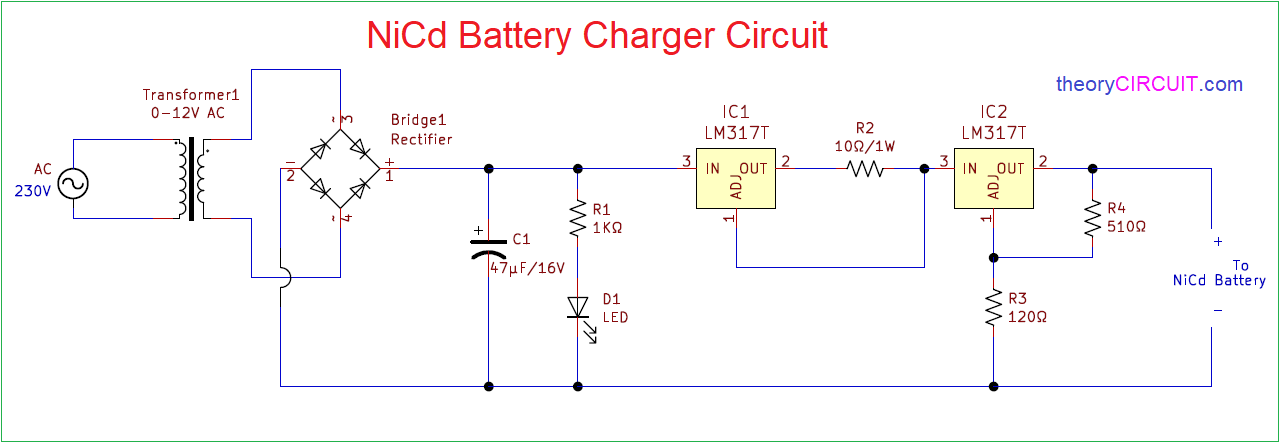
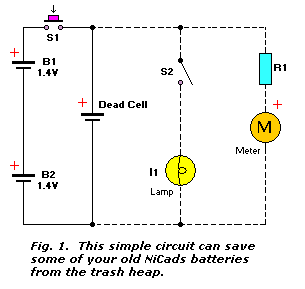
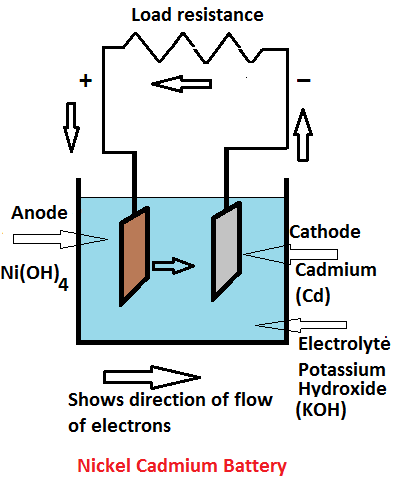
Circuit diagram: Simple NiCd Charger Circuit Diagram. When the cells are almost fully charged, the current gradually drops, so the current limiter becomes inactive and the LED goes out. In this state, the voltage on the reference lead of the regulator depends only on voltage divider R1/R2. For a 7805 regulator, the value of R2 is selected such ... Commercial nickel cadmium (Ni-Cd) batteries weren't popularized until the 1960s by Sanyo in Japan and the United States. Since then, Ni-Cd batteries became very popular for rechargeable home electronics, toys, and power tools. More recently, Nickel Metal Hydride (NiMH) batteries have largely eaten away at their market share. Nickel-Cadmium Battery Operational, Maintenance, and Overhaul Practices. Marathon Batteries P.O. Box 8233 Waco, TX 76714 (254) 776-0650 www.mptc.com. Saft 711 Industrial Boulevard Valdosta ... A nickel-cadmium cell has two plates. The active material of the positive plate (anode) is Ni(OH) 4 and the negative plate (cathode) is of cadmium (Cd) when fully charged. The electrolyte is a solution of potassium hydroxide (KOH) with a small addition of lithium hydrate which increases the capacity and life of the battery.
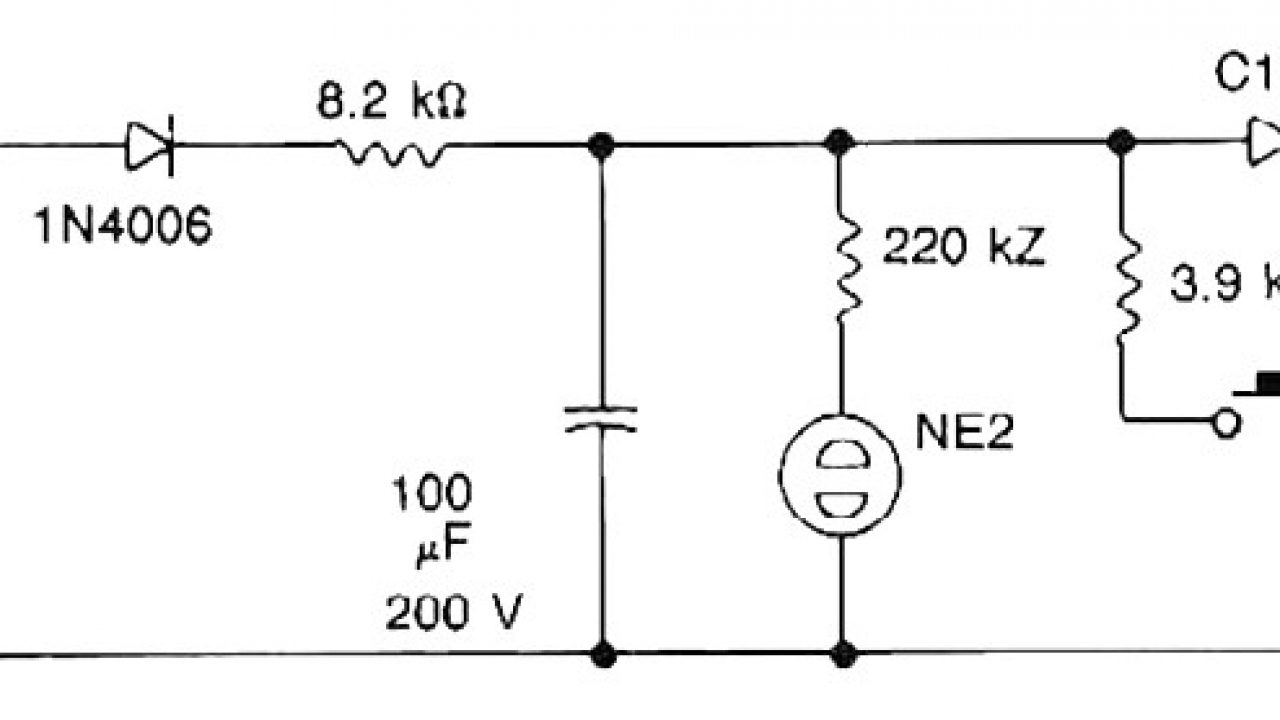
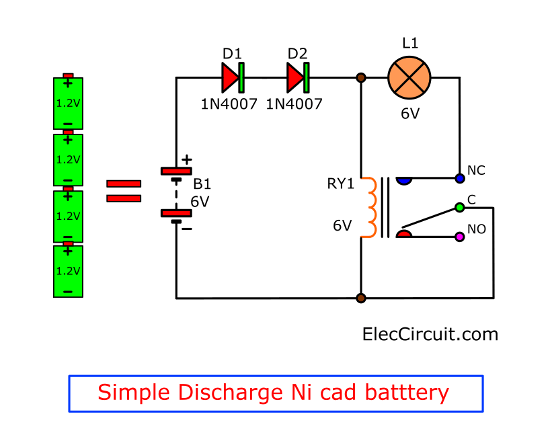
The diagram shows a circuit that is suitable for discharging nickel-cadmium (NiCd) as well as nickel-metal-hydride (NiMH) batteries. It is simple and inexpensive, but the components must be matched to the number of cells the battery contains. As long as D2 lights, the battery is discharged via R4 and T1. When the battery voltage drops below a ... NICKEL-CADMIUM BATTERIES Nickel-cadmium alkaline batteries have gained respect as a very reliable, long life electrochemical system from their performance in (4-1) industrial starter and standby service and in the space program. Space batteries were sintered plate type cells, • Significantly higher capacity than nickel-cadmium batteries. • Typical expectancy life is 2 to 5 years. • Operates well at a wide range of temperatures: Charging 0° C to 50° C Discharging 0° C to 50° C . Battery Description . The nickel-metal hydride battery chemistry is a hybrid of the proven positive electrode chemistry of the sealed Download scientific diagram | Advantages and disadvantages of nickel cadmium batteries from publication: Lifecycle Cost Analysis of Hydrogen Versus Other Technologies for Electrical Energy Storage ...
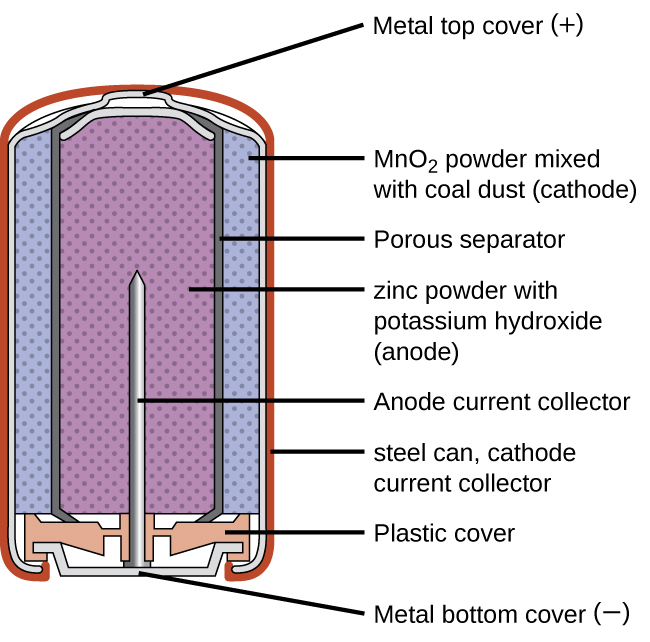
Nickel-Cadmium Battery Diagram. As shown, in the diagram, the nickel acts as a positive electrode collector and the cadmium layer acts as a negative layer collector. The separator layer between the two layers is made up of KOH or NaOH. Its purpose is to provide OH ions. Apart from these, it also consists of a safety valve, sealing plate ...
Today we are going to build charger circuit for charging Ni-Cd Battery. The process of charging Nickel cadmium batteries can be done in two ways: Fast charging; Slow charging; The Fast charging requires a proper shutdown after full charge. Unlike a Lead acid battery or a lithium battery, Ni-cd battery cannot take up a float charge.
Hydrogen and oxygen gases are generated in explosive proportion while the nickel-cadmium battery is being. charged and discharged. Charge the nickel-cadmium battery in a well-ventilated area to reduce concentrations of explosive gases. Turn off the battery charger before connecting or disconnecting the nickel-cadmium battery to pre-vent arching.
2,000 cycles. Nominal cell voltage. 1.2 V. The nickel-cadmium battery ( Ni-Cd battery or NiCad battery) is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes. The abbreviation Ni-Cd is derived from the chemical symbols of nickel (Ni) and cadmium (Cd): the abbreviation NiCad is a registered trademark ...
Nickel-cadmium batteries. Nickel-cadmium batteries at the 1-2h rate yield twice the energy density of lead-acid batteries, i.e. about 50 W-h/kg. They operate over a wide temperature range, give approximately 2000 cycles, and can be charged in less than 1 h. Nickel-cadmium batteries are used in various developmental electric vehicles.
Nickel Iron Wet Nickel Cadmium (NiCd) Vehicle Batteries. These are more user-friendly and a less complicated version of the industrial batteries. They are specifically designed to power cars, motorcycles, boats & other vehicles. An important example of a vehicle battery is the Lead-acid battery. Primary Cell
Difference Between difference between lead acid and nickel cadmium batteries are Following :-. If you have any Query Ask your Questions. Lead acid : Dilute H2SO4, spongy and PbSO2, 1.low efficiency, 2.2 volt per cell, 3.less strong, 4.requires more maintenance, 5.less efficient w.r.t temperature,
From the diagram, the Nickel Cadmium battery energy range from 40 Wh/Kg to 50 Wh/Kg approximately. POWER DENSITY: The power density is expressed in watts per kilogram at cell level. The higher the power density the higher the current that a battery can drive through a given load in a short time.
Nickel-Cadmium Battery. The nickel-cadmium battery system still uses the same positive electrode as the nickel-iron one, while the negative electrode is cadmium. The maximum cell voltage during charge is 1.3 V, and the average cell voltage is 1.2 V. In eqns [4]-[6], the cell reactions during charging and discharging are presented.
A nickel cadmium battery converts chemical energy to electrical energy upon discharge and converts electrical energy back to chemical energy ...
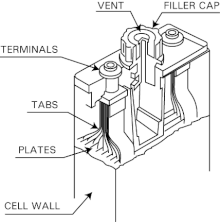
Stationary Nickel-Cadmium Batteries with FNC cells (Batteries / Racks / Cabinet) It is assumed that only qualified person-nel are engaged in assembly and instal-lation of the components provided. Qualified personnel are persons who, on the strength of their training, expe-rience and instruction, together with their knowledge of the relevant stan-
We know the nickel-cadmium battery is called as NiCd battery or NiCad battery and this types of rechargeable batteries are made with nickel oxide hydroxide and metallic cadmium as electrodes. NiCd batteries provides better performance in compact size. Hence it is widely used in hand held and compact electronic devices.
Nickel-Cadmium Battery. The nickel-cadmium (NiCd) battery is another common secondary battery that is suited for low-temperature conditions with a long shelf life. However, the nickel-cadmium batteries are more expensive and their capacity in terms of watt-hours per kilogram is less than that of the nickel-zinc rechargeable batteries.
Nickel-cadmium batteries generally require a constant current charging. The below shown NiCad charger circuit is developed to supply either 50mA to four 1.25V cells (type AA), or 250mA to four 1.25V cells (type C) connected in series, eventhough it could simply be modified for various other charging values.
... Nickelcadmium technology is based on cathode made from nickel oxide hydroxide and anode made from metallic cadmium while electrolyte used for Ni-Cd ...
A nickel-cadmium battery is a system that generates DC voltage by a chemical reaction between the components. In a nickel-cadmium battery, the redox material serves as the nucleus, with a nickel sheet and a separator surrounding it. The voltage of the nickel-cadmium cell is about 1.2 V. As three or four cells are connected in sequence, the output voltage ranges from 3.6 to 4.8 V.
shorts the cell Nickel cadmium battery diagram, courtesy of Radio Shack. 9 Anatomy of a NiCd cell, cntd •Approximately 10-year-old Sanyo Cadnicacell •Found no apparent metallic formations during optical inspection. 10 Experiment #1: Running an electrochemical cell
Nickel (hydroxide)–cadmium systems are the most common small rechargeable battery type for portable appliances. The sealed cells are equipped with “jelly roll” ...

















0 Response to "38 nickel cadmium battery diagram"
Post a Comment