44 draw the molecular orbital diagram shown to determine which of the following is most stable.
[Answered] Use the molecular orbital diagram shown to determine which ... The theory uses Molecular Orbital (MO) diagram to explain the bonding between atoms. For example, to determine the stability of an atom, bond order is calculated. Bond order is calculated to determine the strength and length of the bond. The high value of the bond order means the bond is shorter, stronger, and have high bond strength. 7.7 Molecular Orbital Theory - Chemistry Fundamentals Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
(Get Answer) - Draw the molecular orbital diagram shown to determine ... Draw the molecular orbital diagram shown to determine which of the following is most stable. _____ F22- Ne22+ O22+ F22+ F2 ... Use the molecular orbital diagram shown to determine which of the following paramagnetic. Ne_2^2+ O_2^2+ F_2^2+ O_2^2- None of the above are paramagnetic.

Draw the molecular orbital diagram shown to determine which of the following is most stable.
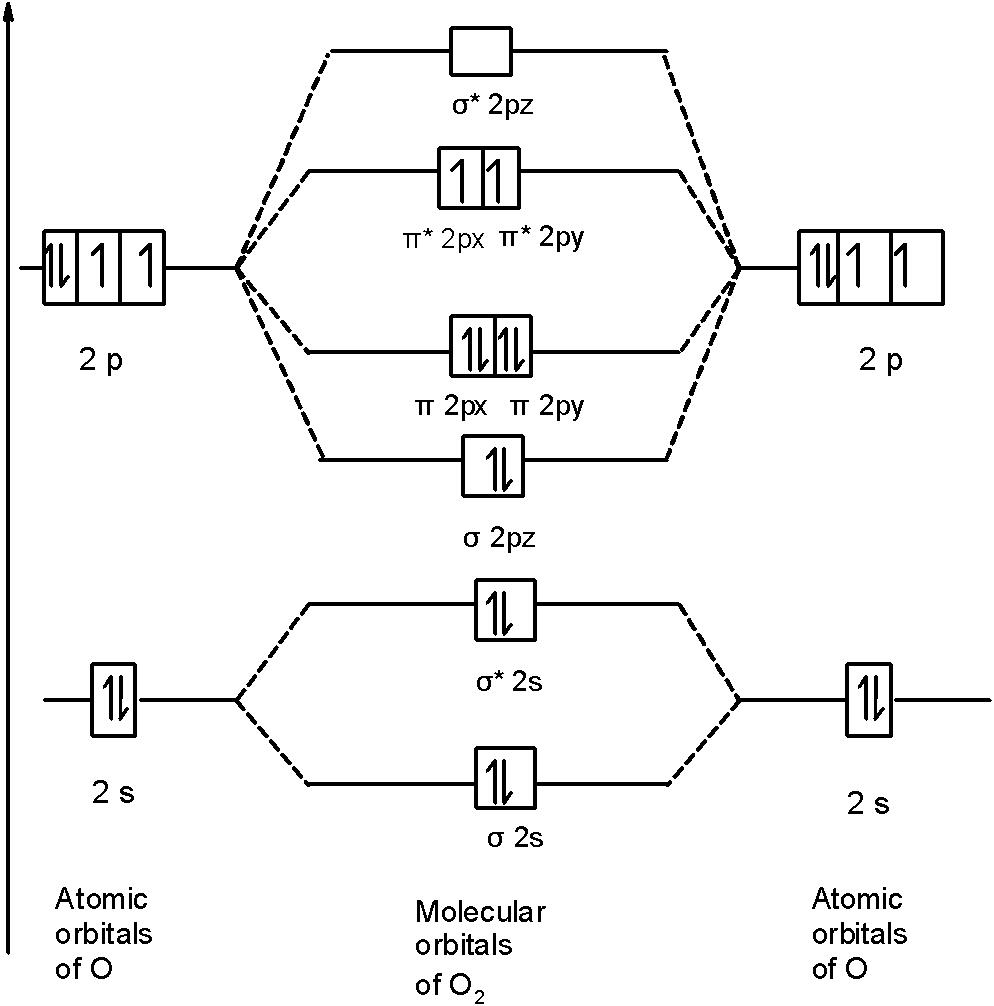
Molecular Orbital Theory | Chemistry I | | Course Hero Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. Use the molecular orbital diagram shown to determine - Course Hero A molecule is stable when bond order is high. Bond order is high when more electron is in bonding molecular orbital. O 2 2+ has only 10 electrons, so the most electrons are in bonding molecular orbital with bond order 3. So, the molecule is most stable. Answered: Draw the molecular orbital diagram… | bartleby Consider these following ions: O2-, N22-, Li2+ and O22- a. Based on molecular orbital theory (MOT), which of the ion(s) exhibit(s) paramagnetism? b. For those ions that are paramagnetic, determine the number of unpaired electrons. Support your answers with draw appropriate molecular orbital energy diagram.
Draw the molecular orbital diagram shown to determine which of the following is most stable.. chemistry 1a Chapter 10 Flashcards | Quizlet Electron groups around the central atom will be most stable when they are as far apart as possible. 2 electron groups have.... groups around the central atom, occupy positions oppiste from each other linear geometry. The bond angle is 180°. 3 electron groups have..... Chemical bonding Flashcards | Quizlet 59) Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) O2^2−. B) Ne2^2+. C) O2^2+. D) F2^2+. E) None of the above are paramagnetic. D) F2^2+. 60) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+. C22- Molecular Orbital Diagram This lowest energy orbital is . Solution: Use the molecular orbital diagram shown to determine which of the following is most stable. A) N22+ B) B2 C) B22+ D) C E) C22+. Sketch the molecular orbital energy level diagram for the ion. How many net σ and π bonds does the ion have? What is the carbon-carbon bond order? Answered: Draw the molecular orbital diagram… | bartleby Draw the molecular orbital diagram shown to determine which of the following is most stable. B2, N2^2+, C2^2-, B2^2+, C2^2+ Question Draw the molecular orbital diagram shown to determine which of the following is most stable. B2, N2^2+, C2^2-, B2^2+, C2^2+ Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution
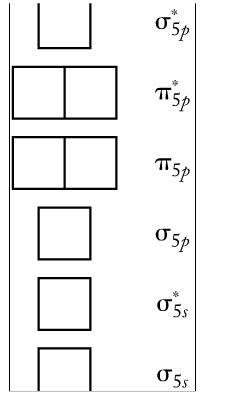
Molecular Orbital Theory - Chemistry - University of Hawaiʻi The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The net contribution of the electrons to the bond strength of a molecule is identified by determining the bond order that results from the filling of the molecular orbitals by electrons. Quiz 1 Flashcards | Quizlet Molecular Orbital Diagram Molecular Orbital Theory Molecular Orbital Terms in this set (40) Which of the following is true? A) a bond order of 0 represents a stable chemical bond B) electrons placed in antibonding orbitals stabilize the ion/molecule Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+ B) N2^2+ C) B2 D) C2^2- E) B2^2+ 5) Which statement regarding stable heteronuclear diatomic molecules is false? A) All have bond orders greater than zero. Answered: 1 -Use the molecular orbital diagram… | bartleby Q: Use the Molecular Orbital Diagram to the following species: 1) O2 2) 0,2+ 3) F2 4) F22+. A: Explanation Molecular Orbital Theory: Atomic orbitals are hybridized to form molecular orbitals. The…. Q: The research group has recently focused on identifying the chemical properties of fluorocarbons,….
Molecular Orbital Theory | Chemistry | | Course Hero Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules with more complicated bonding (beyond the scope of this text) that are difficult to describe with Lewis structures. Chem Flashcards - Quizlet Give the approximate bond angle for a molecule with an linear shape. A) 109.5° B) 180° C) 120° D) 105° E) 90° b Determine the electron geometry (eg) and molecular geometry (mg) of SiF4. A) eg=tetrahedral, mg=trigonal pyramidal B) eg=octahedral, mg=square planar C) eg=trigonal bipyramidal, mg=trigonal pyramidal D) eg=tetrahedral, mg=bent Chemistry 2 Final Exam Flashcards - Quizlet Draw the molecular orbital diagram shown to determine which of the following is most stable. O22, C22- Choose the compound below that contains at least one polar covalent bond, but is nonpolar. Solved Draw the molecular orbital diagram shown to determine - Chegg This problem has been solved! See the answer Draw the molecular orbital diagram shown to determine which of the following is most stable. N22+ C22+ C22- B22+ B2 Expert Answer 100% (36 ratings) C22- should have the highest bond order (3, it has 6 more e … View the full answer Previous question Next question
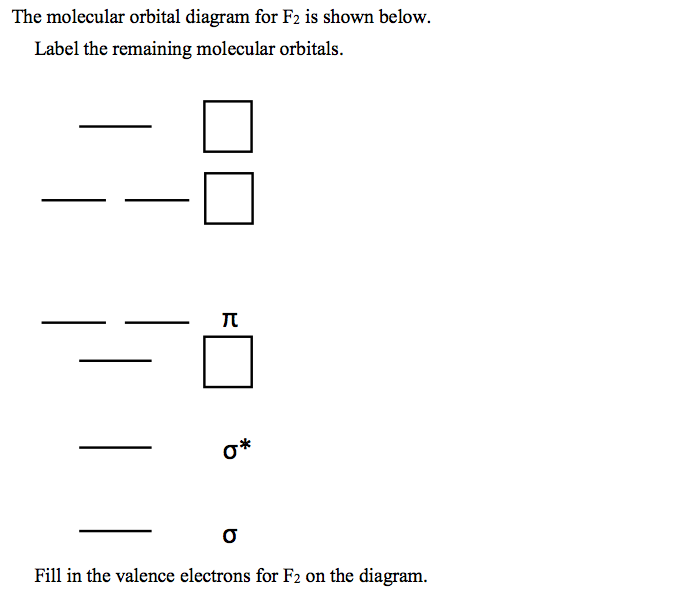
Solved Draw the molecular orbital diagram shown to determine - Chegg Question: Draw the molecular orbital diagram shown to determine which of the following is most stable. F22- Ne22+ O22+ F22+ F2 This problem has been solved! See the answer Draw the molecular orbital diagram shown to determine which of the following is most stable. F22- Ne22+ O22+ F22+ F2 Best Answer 100% (12 ratings)
Mock Final - GitHub Pages 31) Use the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2⁺ B) N2^2⁺ C) B2; D) C2^2⁻ E) B2^2⁺ 32) How many moles of oxygen are formed when 58.6 g of KNO3 decomposes according to the following reaction? The molar mass of KNO3 is 101.11 g/mol. 4 KNO3(s) → 2 K2O(s) + 2 N2(g) + 5 O2(g)
8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
Solved Draw the molecular orbital diagram shown to determine - Chegg Question: Draw the molecular orbital diagram shown to determine which of the following is most stable. Draw the molecular orbital diagram shown to determine which of the following is most stable. F22+ F2 Ne22+ O22+ F22- This problem has been solved! See the answer
Answered: Draw Lewis structures and MO diagrams… | bartleby Q: 1. Sketch the MO diagram of the following species and fill the molecular orbitals with electrons.…. Q: Draw the Lewis structure for C2H2 . Is the molecule polar or nonpolar? What is the electronic…. Q: Fill in the blanks: Given the following: C2, C2*, C2 According to the MO theory has the longest bond….
Answered: Use molecular orbital energy diagrams… | bartleby Solution for Use molecular orbital energy diagrams to determine which of the following is most stable (has highest bond order). (A) C22+ (B). N22+ (C). B2…
Answered: Draw the molecular orbital diagram… | bartleby Consider these following ions: O2-, N22-, Li2+ and O22- a. Based on molecular orbital theory (MOT), which of the ion(s) exhibit(s) paramagnetism? b. For those ions that are paramagnetic, determine the number of unpaired electrons. Support your answers with draw appropriate molecular orbital energy diagram.
Use the molecular orbital diagram shown to determine - Course Hero A molecule is stable when bond order is high. Bond order is high when more electron is in bonding molecular orbital. O 2 2+ has only 10 electrons, so the most electrons are in bonding molecular orbital with bond order 3. So, the molecule is most stable.


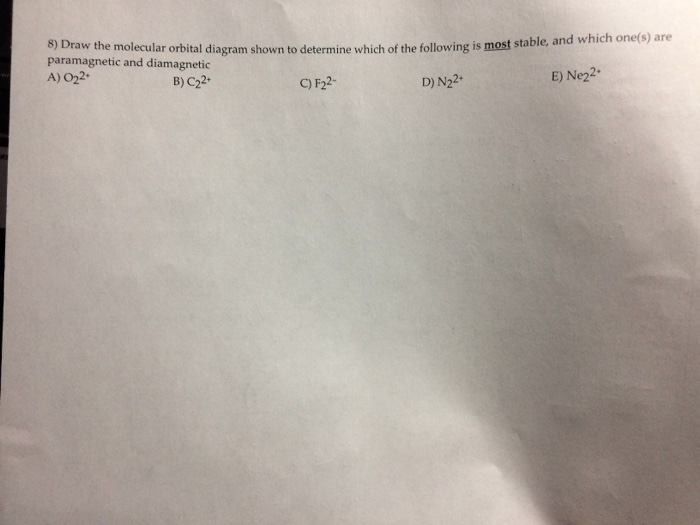

0 Response to "44 draw the molecular orbital diagram shown to determine which of the following is most stable."
Post a Comment