43 full orbital diagram for n
Answered: What is the orbital diagram for the… | bartleby Want to see this answer and more? Experts are waiting 24/7 to provide step-by-step solutions in as fast as 30 minutes!*. *Response times may vary by subject and question complexity. Median response time is 34 minutes for paid subscribers and may be longer for promotional offers. Hund's Rule and Orbital Filling Diagrams | Chemistry for Non-Majors ... According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Since the s sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium. The next element is lithium and necessitates the use of the next available sublevel, the 2s.. The filling diagram for carbon is shown in the Figure below.
Answered: Write the full orbital diagram for… | bartleby We've got the study and writing resources you need for your assignments.Start exploring!

Full orbital diagram for n
Orbital filling diagrams | The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... s,p,d,f Orbitals - Chemistry | Socratic Not all electrons inhabit s orbitals. At the first energy level, the only orbital available to electrons is the 1s orbital. However, at the second level, there are also orbitals called 2p orbitals in addition to the 2s orbital. Unlike an s orbital, a p orbital points in a particular direction. The one shown below points up and down the page. How to Draw Orbital Diagrams - YouTube Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
Full orbital diagram for n. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... Electron configuration for Niobium (element 41). Orbital diagram The order of filling the orbitals with electrons in the Nb atom is an exception to the rule. Expected electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 3 But in reality, one electron moves from the 5s orbital to the 4d orbital: Electronic configuration of the Niobium atom in ascending order of orbital energies: Solved Write the full orbital diagram for He. Drag the | Chegg.com Reset Help 15 25 G1 G1 G1 GG GI G1 GI G1 G2 G2 G2 G2 G2 2p 3s 3p Pearson Write the full orbital diagram for N. Drag the appropriate labels to their respective targets, Labels can be used once, more than once, or not at all. Not all targets will be filled. Reset Help 11 1s 28 GI GI ar GIGI GIGIO ale 2p GP GR G2 GP 35 3p Pearson Orbital Diagrams and Electron Configuration - YouTube This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
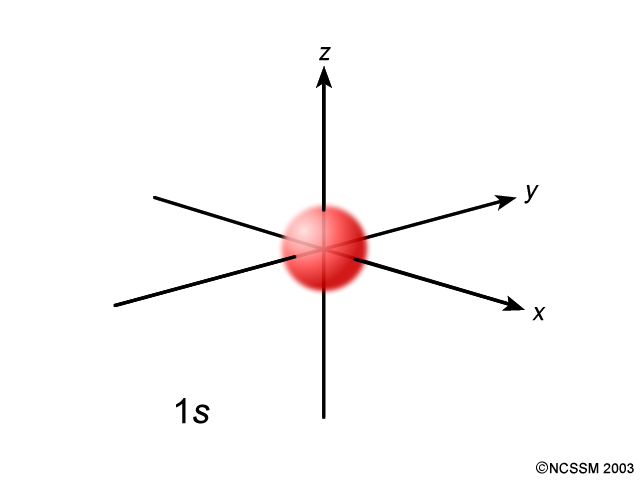
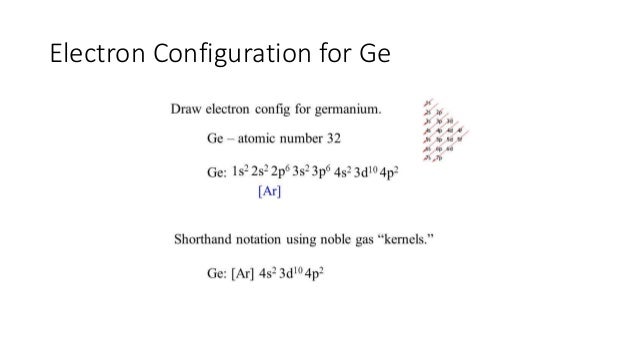
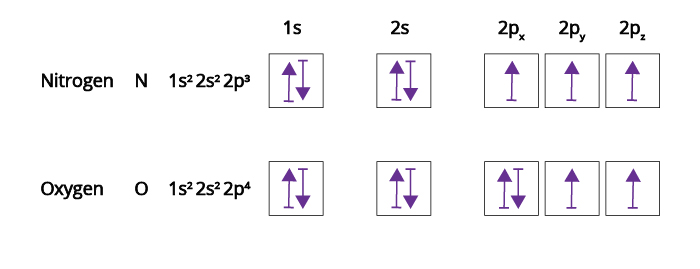
Atomic orbital - Wikipedia In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus.The term atomic orbital may also refer to the physical region or space where the electron can be calculated to ... Nitrogen(N) electron configuration and orbital diagram n=4 for N orbit. The maximum electrons holding capacity in N orbit is 2n 2 = 2 × 4 2 = 32. Therefore, the maximum electron holding capacity in the first shell is two, the second shell is eight and the 3rd shell can have a maximum of eighteen electrons. The atomic number is the number of electrons in that element. Neodymium(Nd) electron configuration and orbital diagram Then the remaining four electrons will enter the 4f orbital. Therefore, the neodymium full electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 4 5s 2 5p 6 6s 2. Neodymium electron configuration. Note: The short electron configuration of neodymium is [ Xe] 4f 4 6s 2. Neon(Ne) electron configuration and orbital diagram The first two electrons of neon enter the 1s orbital and the next two electrons enter the 2s orbital. The s-orbital can have a maximum of two electrons. So, the remaining six electrons enter the 2p orbital. Therefore, the neon full electron configuration will be 1s 2 2s 2 2p 6. Note: The short electron configuration of neon is 2s 2 2p 6. When writing an electron configuration, you have to write serially.
Sodium(Na) electron configuration and orbital diagram The atomic number of an element is the number of electrons and protons in that element. That is, the number of electrons and protons in the sodium atom is eleven. The sodium electron configuration is 1s 2 2s 2 2p 6 3s 1. The active atomic mass of the sodium atom is 22.98976928. Sodium is an alkali metal. Answered: Write the full orbital diagram for each… | bartleby Solution for Write the full orbital diagram for each element.a. N b. F c. Mg d. Al Oxygen(O) electron configuration and orbital diagram Therefore, the next two electrons enter the 2s orbital. The p-orbital can have a maximum of six electrons. So, the remaining four electrons enter the 2p orbital. Therefore, the oxygen full electron configuration will be 1s 2 2s 2 2p 4. Note: The short electron configuration of oxygen is [ He] 2s 2 2p 4. Electron Configurations and Orbital Box Diagrams - Pathways to Chemistry An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin-one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below.
Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
SOLVED:Write the full orbital diagram for each element. a. N b. F c. Mg ... Write the full orbital diagram for each element. a. N b. F c. Mg d. Al. Answer. a. diagram not available b. diagram not available c. diagram not available d. diagram not available (SEE SOLUTION) View Answer. Related Courses. Chemistry 101. Chemistry. Chapter 3. Periodic Properties of the Elements.
Write the full orbital diagram for each element. - Numerade Write the full orbital diagram for each element. a. N b. F c… 01:11. Write full orbital diagrams and indicate the number of unpaired electrons fo… 01:10. Write full orbital diagrams and indicate the number of unpaired electrons fo… 02:16. Write the full orbital diagram for each element. ...
Solved Write the full orbital diagram for B, Li, N, | Chegg.com Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. The orbital diagram of Boron, lithium, nitrogen and helium are mentioned above. The unpaired electrons are indicated by red circle in the orbital diagram. We know the atomic numbers of boron is 5 , so it's ...
To give: The full orbital diagram for N . | bartleby To give: The full orbital diagram for N . Question. Chapter 8, Problem 43E (a) Interpretation Introduction. To give: The full orbital diagram for N. (b) Interpretation Introduction. To give: The full orbital diagram for F. (c) Interpretation Introduction. To give: The full orbital diagram for Mg. (d)
Solved 41. Write the full electron configuration for each - Chegg Write the full electron configuration for each element. (a) Si (b) 0 (c) K (d) Ne 43. Write the full orbital diagram for each element. (a) N (b)F (c) Mg (d) Al 45. Use the periodic table to write an electron configuration for each element. Represent core electrons with the symbol of the previous noble gas in brackets. (a) As (b) Sn (c) Ti (d ...
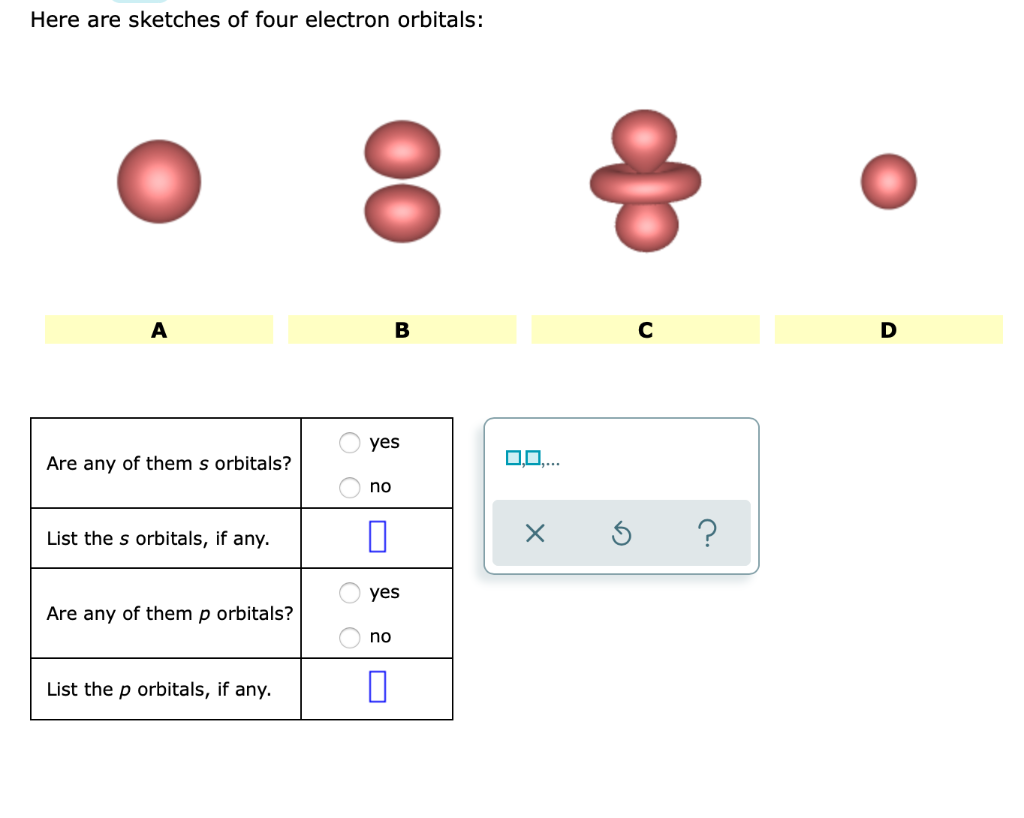
Orbitals Chemistry (Shapes of Atomic Orbitals) - Shape of s, p, d, and ... The size of the s orbital is also found to increase with the increase in the value of the principal quantum number (n), thus, 4s > 3s> 2s > 1s. The Shape of p Orbitals. Each p orbital consists of two sections better known as lobes which lie on either side of the plane passing through the nucleus.
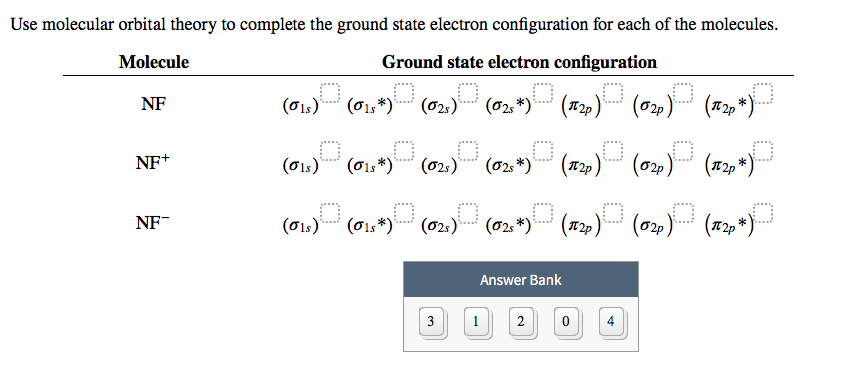
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This tool is very
Orbital Diagrams Chemistry Tutorial - AUS-e-TUTE p z. For example, an atom of nitrogen has 7 electrons ( number of electrons = atomic number = Z = 7) Nitrogen is a period 2 element, and is a p-block element, therefore: The 1 st energy level is full: 1s 2. In the 2 nd energy level the s orbital is full, 2s 2 and there are 3 electrons in the p orbitals, 2p 3.
Write full orbital diagrams and indicate the number of unpaired ... Video Transcript. looking at orbital diagrams for the following elements. And then we'll also probably just discuss de kind of electron pairing whether we have any umpired electrons or or paired electrons and then that will allow us to determine whether we have a dime magnetic or power magnetic species where di magmatic, all of our electrons repaired para magnetic we have unpowered elect ...
How to Draw Orbital Diagrams - YouTube Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
s,p,d,f Orbitals - Chemistry | Socratic Not all electrons inhabit s orbitals. At the first energy level, the only orbital available to electrons is the 1s orbital. However, at the second level, there are also orbitals called 2p orbitals in addition to the 2s orbital. Unlike an s orbital, a p orbital points in a particular direction. The one shown below points up and down the page.
Orbital filling diagrams | The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...

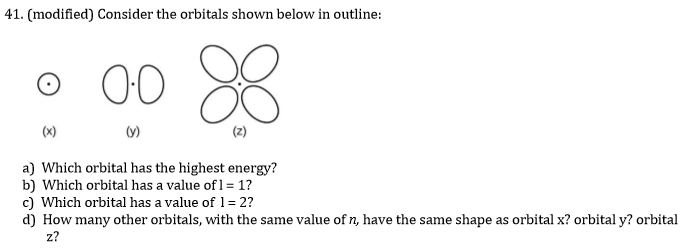
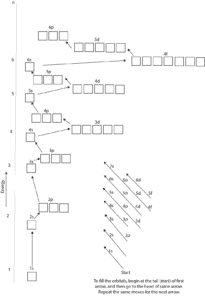


0 Response to "43 full orbital diagram for n"
Post a Comment