44 bromine lewis dot diagram
Bromine Lewis Dot Structure - Drawing Method of Bromine, Lewis ... How many dots should there be in a bromine atom's Lewis symbol? In bromine's atom Lewis electron dot diagram there should be seven dots arranged correctly, as it has seven valence electrons. Why is Br 2 classified as a nonpolar molecule? The bromine molecule has a geometrical structure that is linear. Additionally, it contains two bromine atoms. How to Draw the Lewis Dot Structure for Br ( the Element Bromine) A step-by-step explanation of how to draw the Br Lewis Dot Structure.For the Br structure use the periodic table to find the total number of valence electron...
(PDF) Study Guide and Solutions Manual to Accompany T.W. 30/11/2014 · Study Guide and Solutions Manual to Accompany T.W. Graham Solomons / Craig B. Fryhle / Scott A. Snyder / Jon Antilla
Bromine lewis dot diagram
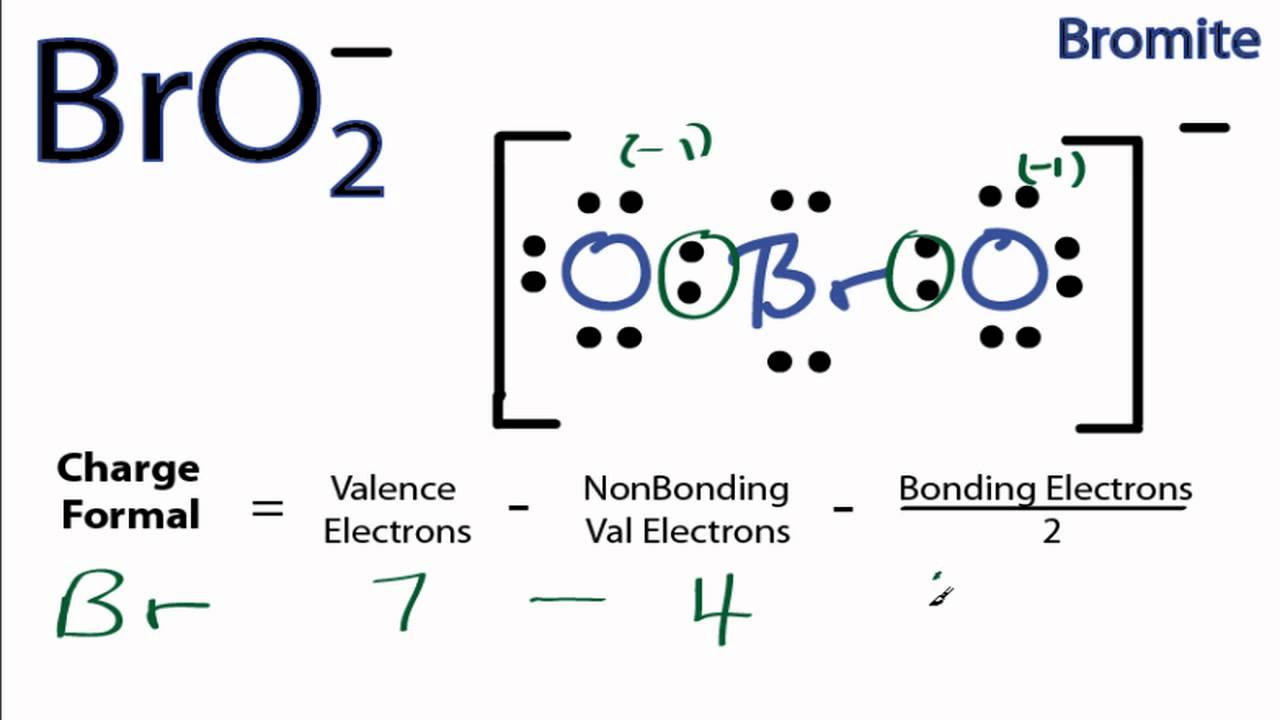
BrF5 lewis structure, molecular geometry, bond angle ... Follow some steps for drawing the lewis dot structure of BrF5 1. Count total valence electron in BrF5 In the very first step, we need to determine how many valence electrons are available for BrF5. A valence electron is the outermost shell electron associated with an atom. It is represented as dots in the lewis diagram. XeF2 Lewis Structure, Molecular Geometry, Hybridization, and ... Jun 15, 2022 · XeF2 Lewis Structure. Lewis Structure, also known as electron dot structure, is an essential model of chemical bonding where we use the valence electron concept to schematically sketch a two-dimensional figure of a given molecule. We use dots to represent outer shell electrons and lines to represent the bond type. Xenon is an inert gas element. BrO3- Lewis Structure - Learnool In the lewis structure of BrO 3-, there is one single bond and two double bonds around the bromine atom, with three oxygen atoms attached to it. The oxygen atom with a single bond has three lone pairs, the two oxygen atoms with double bonds have two lone pairs, and the bromine atom has one lone pair.
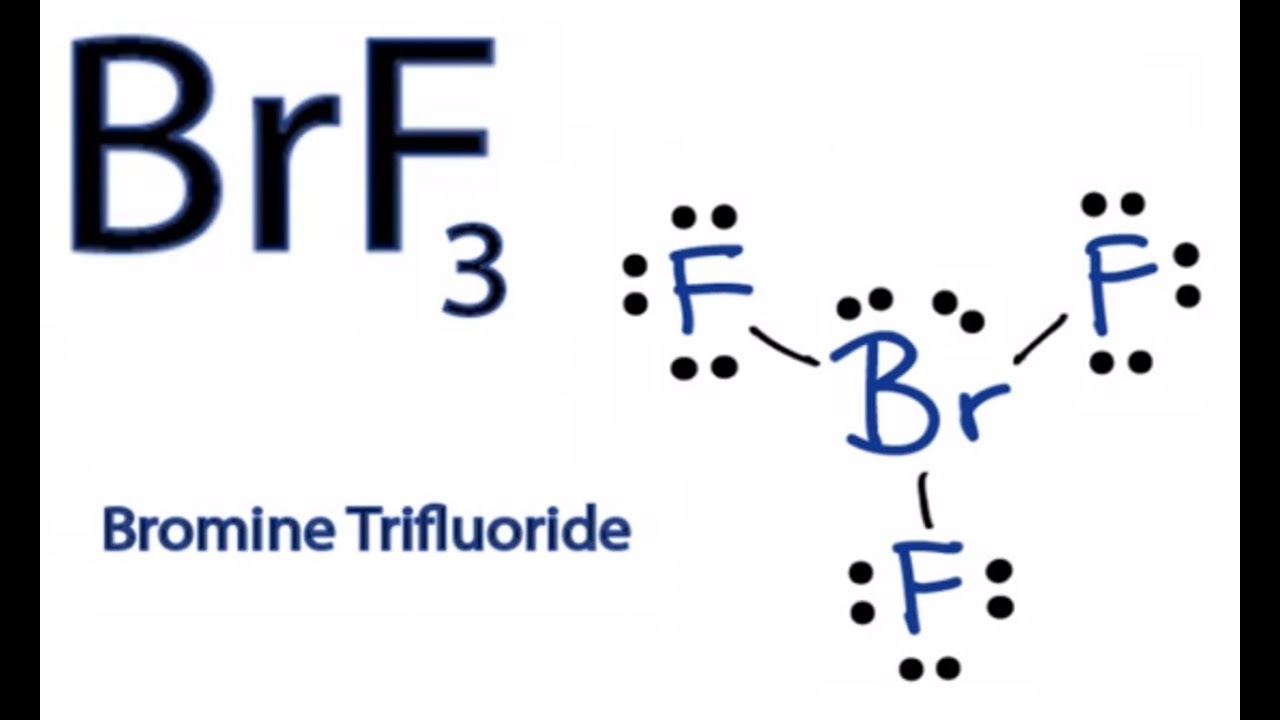
Bromine lewis dot diagram. Bromate ion (BrO3-) lewis dot structure, molecular ... - Topblogtenz Follow some steps for drawing the lewis dot structure for BrO3- 1. Count total valence electron in BrO3- Lewis diagram is a simple representation of valence electron within a molecule. So, for determining the valence electron in BrO3-, look at the periodic group of bromine and oxygen atoms. How to draw HBr Lewis Structure? - Science Education and Tutorials To sketch the HBr Lewis structure by following these instructions: Step-1: HBr Lewis dot Structure by counting valence electrons on the bromine atom. Step-2: Lewis Structure of HBr for counting valence electrons around the terminal hydrogen atoms. Step-3: Lewis dot Structure for HBr generated from step-1 and step-2. How to Draw the Lewis Dot Structure for NaBr: Sodium bromide A step-by-step explanation of how to draw the NaBr Lewis Dot Structure.For NaBr we have an ionic compound and we need to take that into account when we draw ... How to draw BrF3 Lewis Structure? - Science Education and Tutorials It is represented by dots in the BrF3 Lewis diagram. The BrF3 molecule's core bromine atom can be represented as follows: Total outermost valence shell electron of bromine atom in BrF3= 7 Total outermost valence shell electron of fluorine atom in BrF3= 7 The BrF3 molecule has one central bromine and three fluorine atoms.
Lewis Structures: Dot Symbols, Diagram, Examples - Embibe A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds. It defines the nature of the bond and position of atoms of the molecule which are connected in the molecule. The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. PBr3 Lewis Structure, Molecular Geometry, Polarity, and ... Jun 15, 2022 · For Br, the formal charge= 7 – 0.5*2 – 6 = 0 ( holds true for all the three bromine atoms) So, now we put the single bonds to get our perfect Lewis Structure. PBr3 Molecular Geometry. Learning about the lewis structure leads us to our next concept: Molecular Bonding. Definition and Introduction Chem 101 practice final exam - Cal State LA Bromine (Br), Fluorine (F), Sulfur (S), Arsenic (As) 28. (15 points) Draw the Lewis structure of carbonic acid, H 2CO 3, and determine the formal charge of each atom in the structure. 29. (15 points) Draw the molecular orbital energy diagram for nitrogen monoxide, NO, and determine the bond order of the molecule. (Reminder: place the atomic ... Oxidation state - Wikipedia In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic.It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound.Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong …
AP Chemistry 2019 Free-Response Questions - College Board The complete Lewis electron-dot. diagram for the urea molecule is shown above. (a) Identify the hybridization of the valence orbitals of the carbon atom in the urea molecule. (b) Urea has a high solubility in water, due in part to its ability to form hydrogen bonds. A urea molecule and four water molecules are represented in the box below. Draw ONE dashed line (----) to indicate a possible ... How to draw BBr3 Lewis Structure? - Science Education and Tutorials To sketch the BBr3 Lewis structure by following these instructions: Step-1: BBr3 Lewis dot Structure by counting valence electrons on the boron atom. Step-2: Lewis Structure of BBr3 for counting valence electrons around the terminal bromine atom. Step-3: Lewis dot Structure for BBr3 generated from step-1 and step-2. Inorganic Chemistry by Miessler ~ 5th Edition - Academia.edu This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. Brf3 Lewis Structure: Draw the Bromine Trifluoride Dot Structure So to understand the Lewis Dot structure of BrF3, let's first know some basic details needed to make this structure. BrF3 Valence Electrons Bromine has seven electrons in its valence shell, and fluorine also has seven electrons in its outer shell. To get the total number of valence electrons, we have to add all these electrons: Br = 7 electrons
Lewis dot diagram for bromine? - Answers All the halogens atoms, Fluorine , chlorine ,iodine and Astatine have the same dot diagram as the bromine. How many dots would a Lewis dot diagram for Boron have? The Lewis dot diagram for Boron ...
3.1 Chemical bonds | Atomic combinations | Siyavula A Lewis diagram uses dots or crosses to represent the valence electrons on different atoms. The chemical symbol of the element is used to represent the nucleus and the core electrons of the atom. The chemical symbol of the element is used to represent the nucleus and the core electrons of the atom.
Br2 Lewis Structure - How to Draw the Lewis Dot Structure for ... - YouTube Br2 is also called Bromine gas. ----- Steps to Write Lewis Structure for compounds like Br2 ----- 1. Find the total valence electrons for the Br2 molecule. 2. Put the least electronegative atom in...
Nitrogen tribromide (NBr3) lewis dot structure, molecular geometry ... The electronegativity of nitrogen and bromine atom is very near to each other, so, the other method to determine the central atom of the lewis diagram is by observing the least numerous element in the compound. The element which repeats least in the compound should be the central atom in the lewis diagram. So, in the case of NBr3, the nitrogen atom repeated only one time …
Lewis Electron Dot Diagrams – Introductory Chemistry – 1st ... A. Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
How to draw BrF5 Lewis Structure? - Science Education and Tutorials It is represented by dots in the BrF5 Lewis diagram. The BrF5 molecule's core bromine atom can be represented as follows: Total outermost valence shell electron of bromine atom in BrF5= 7 Total outermost valence shell electron of fluorine atom in BrF5= 7 The BrF5 molecule has one central bromine and five fluorine atoms.
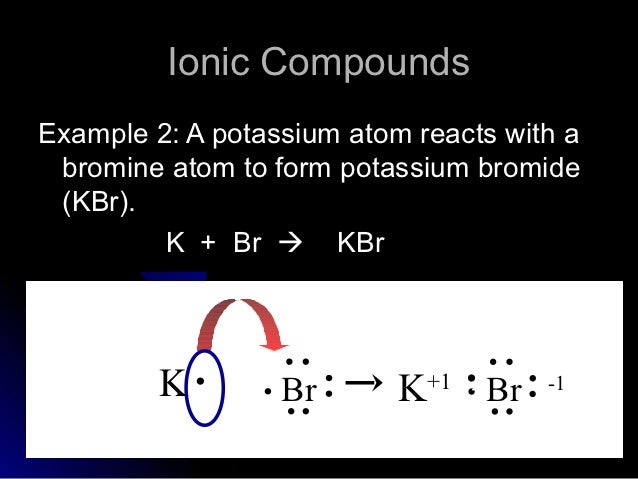
Chapter 9 Section A Lewis Electron Dot Diagrams - PEOI Draw the Lewis electron dot diagram for each element. bromine gallium 9. Draw the Lewis electron dot diagram for each ion. Mg 2+ S 2- Mg 2+ 10. Draw the Lewis electron dot diagram for each ion. In + Br - 11. Draw the Lewis electron dot diagram for each ion. Fe 2+ N 3- Fe 2+ 12. Draw the Lewis electron dot diagram for each ion. H + H - H +
Lewis Electron Dot Diagrams – Introductory Chemistry – 1st … Problem. What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: . The valence electron configuration for selenium is 4s 2 4p 4.In the highest-numbered shell, the n = 4 shell, …
NBr3 lewis structure, molecular geometry, bond angle ... The total valence electron available for the NBr3 lewis dot structure is 26. The hybridization of NBr3 is Sp³. Nitrogen tribromide is slightly polar in nature. The molecular geometry of NBr3 is trigonal pyramidal and its electron geometry is tetrahedral. Lewis structure of NBr3 contains 1 lone pair and 3 bonded pairs.
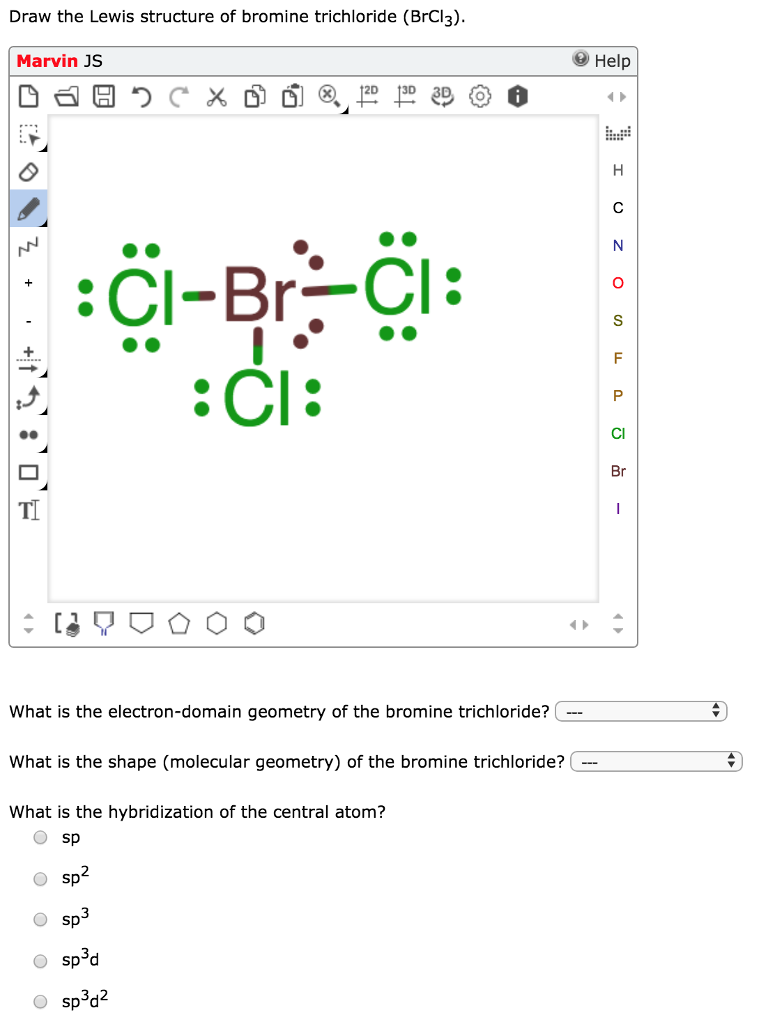
How to Draw the Lewis Dot Structure for BrCl: Bromine ... - YouTube BrCl is also called Bromine monochloride. ----- Steps to Write Lewis Structure for compounds like BrCl ----- 1. Find the total valence electrons for the BrCl molecule. 2. Put the least...
Bromine Triiodide BrI3 Lewis Dot Structure - YouTube A video explanation of how to draw the Lewis Dot Structure for Bromine Triiodide, along with information about the compound including Formal Charges, Polarit...
Bromine Lewis Dot Structure: Drawing, Several Compounds and Detailed ... Bromine Lewis dot structure (Bromide ion) Bromine (Atomic number = 35 and electronic configuration = 2,8,18,7) belongs to group 17 of the periodic table. So it has 7 valence electrons that are involved in chemical bond formation. So to achieve its octet stability it needs to gain 1 electron.
lewis dot - All about bromine Who discovered Bromine? The atomic mass and atomic number of bromine; Physical properties of Bromine; Bromine's location in nature; Melting and boiling points of Bromine; Other elements in the group of Bromine; Type of element; Compounds it is used in ; Uses for Bromine; Unique info for bromine; location ; Bohr Diagram; lewis dot; Aufbou; Sources
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry For example, the Lewis electron dot diagram for calcium is simply. Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as …
Br2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Its structure is Linear. A quick way to learn the concept of the hybridization model is as follows: Step 1: Check the central atom and count the atoms connected with it. Step 2: Next step is to count the number of lone pairs. Step 3: Now, let us add these numbers together. If the count is 4, the composition is SP3.






0 Response to "44 bromine lewis dot diagram"
Post a Comment