38 progress of reaction diagram
PDF Representing a Reaction with a Potential Energy Diagram the x- axis follows the reaction progress. The reactants will be shown as the first level portion, and the products will be the second level portion of the diagram. The ... Draw a potential energy diagram for the reaction showing appropriate labels for both axes, E a(fwd), E a(rev), and H r. Energy profile (chemistry) - Wikipedia For a chemical reaction or process an energy profile (or reaction coordinate diagram) is a theoretical representation of a single energetic pathway, along the reaction coordinate, as the reactants are transformed into products.
Potential Energy Diagrams ( Read ) | Chemistry - CK-12 Foundation Explains potential energy diagrams and activation energy. ... Graphs of the energy changes that occur during a chemical reaction. % Progress . MEMORY METER. This indicates how strong in your memory this concept is. Practice. Preview; Assign Practice; Preview. Progress % Practice Now.
Progress of reaction diagram
Reaction coordinate - Wikipedia In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. In molecular dynamics simulations, a reaction coordinate is called collective variable. PDF Potential Energy vs Reaction Progress (Coordinate) Diagram On your sketch, label the position of reactants, products, any intermediates, any activated complexes (transition states), activation energy for the RDS and ∆H for the first step and the overall reaction. Suggest a diagram for the same reaction, which occurs in the absence of a catalyst. Reaction progress kinetic analysis - Wikipedia Reaction progress data may often most simply be presented as plot of substrate concentration ( [A] t) vs. time ( t) or fraction conversion ( F) vs. time ( t ). The latter requires minor algebraic manipulation to convert concentration/absorbance values to fractional conversion ( F ), by: F = [A] 0 − [A] t [A] 0
Progress of reaction diagram. Calculating energies for a reaction progress diagram using ... - YouTube Here Professor Neshyba shows how to construct a quantitative reaction progress diagram for the reaction of two Hydrogen atoms to form H2. He shows how to bui... Reaction Profiles - Michigan State University Since different types of bonds have different bond strengths, the energy of the reacting system changes as the reaction progresses. Graphs that show the change in energy as a function of the progress of the reaction are known as reaction coordinate diagrams or reaction profiles. In this topic we will look at the most common types of reaction ... Potential Energy Diagrams - Kentchemistry.com In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. This state is also known as an activated complex. Effect of a Catalyst Reaction profiles - Exothermic and endothermic reactions - AQA - GCSE ... Reaction profiles An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them....
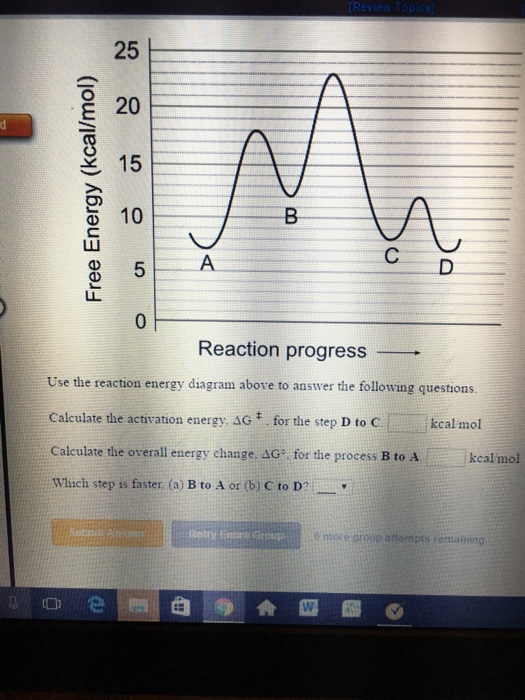
Solved - --- Free Energy - Progress of the Reaction - Chegg.com Expert Answer 100% (1 rating) Answer=D Explanation=D represent the free energy change for the reaction with out the enzyme. N … View the full answer Transcribed image text: - --- Free Energy - Progress of the Reaction - The energy diagram above shows a reaction in the presence and absence of an enzyme. ActivationEnergyWorksheet.docx - Activation Energy... Use the Progress of reaction diagrams below to answer the questions a - e below: a) Is Reaction 1 below endothermic/ exothermic ? b) Which reaction below is the activation energy greater in the reverse reaction? Reaction 1/ Reaction 2. Reaction 1 / Reaction 2. Representing endothermic and exothermic processes using energy diagrams ... The peaks in energy diagrams for both endothermic and exothermic reaction energy diagrams are known as the transition state or the activation complex. In a reaction, any reaction, the same general trend occurs. First the bonds of the reactants are broken which requires an input of energy to be put into the reaction. Potential Energy Diagrams - Chemistry - YouTube This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Solved 6) Reaction Progress Diagram - Draw a reaction | Chegg.com 6) Reaction Progress Diagram - Draw a reaction progress diagram for a reaction with a free energy of reaction of -33.0 kJ/mol and an activation energy of 25.0 kJ/ mol. Label the diagram with the forward and reverse activation energies and the transition state. What is the activation for the reverse reaction? kJ/mol Evaluate Change Problem Values 16.3a Interpreting a reaction energy diagram - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... 6.6: Reaction Coordinate Diagrams - Chemistry LibreTexts You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the 'reaction coordinate', tracing from left to right the progress of the reaction from starting compounds to final products. PDF Chemistry 12 - cpb-ca-c1.wpmucdn.com 24. Burning coal (Carbon) is a highly exothermic reaction. However coal, in contact with air at room temperature has such a slow reaction that it is not noticeable. Explain these two facts with the help of a Potential Energy Diagram Potential Energy (KJ) Progress of Reaction Worksheet 1-2 Potential Energy Diagrams Page 4
Energy Diagrams, Transition States, and Reactive Intermediates ... - JoVE The reaction coordinate indicates the progress of the conversion of reactants to products. Peaks on the energy diagram represent the transition states, whereas the valleys represent the reactive intermediates. As the reaction progresses, the reactants pass through an unstable state of maximum free energy, called the activated complex, or the ...
Answered: a) Draw the reaction coordinate diagram… | bartleby a) Draw the reaction coordinate diagram (reaction progress vs. energy) that is consistent with this mechanism. Assume the reaction is exothermic. Label where is ES appears in the diagram. The Michaelis-Menten equation gives the rate law for this mechanism: Rate = k2 [E]o [S]/ (Ka + [S]) b) With an understanding of this rate law, identify which ...
Reaction Coordinate Diagrams The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction (heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
Analyzing Energy With a Reaction Coordinate Diagram - Study.com A reaction coordinate diagram is a diagram that plots energy versus reaction progress. 3.5K views Explaining the Reaction Coordinate Diagram So let's look a little closer at the reaction coordinate...
The following diagrams show the progress of the reaction 2A | Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: The following diagrams show the progress of the reaction 2A $\to$. Determine whether the reaction is first order or second order and calculate the rate constant. t = 0 min : 16 A, 0 A2 t = 15 min: 8 A, 4 A2 t = 30 min: 4 A, 6 A2.
Energy Diagrams - Concept - Chemistry Video by Brightstorm Energy diagrams depict the reaction progress versus energy. For exothermic reactions, the reactants are drawn above the products because their energy is greater. However, for endothermic reactions, the reactants are drawn below the products. Catalysts lower activation energy, so they decrease the size of the hump within the diagram itself.
The following diagrams represent the progress of the reactio | Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: The following diagrams represent the progress of the reaction A$\to$ B where the red spheres represent A molecules and the green spheres represent B molecules. Calculate the rate constant of the reaction. t = 0 s: 12 A t= 20 s: 6 A, 6 B t=4 s: 3 A, 9 B.
Energy Diagrams of Reactions | Fiveable Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.

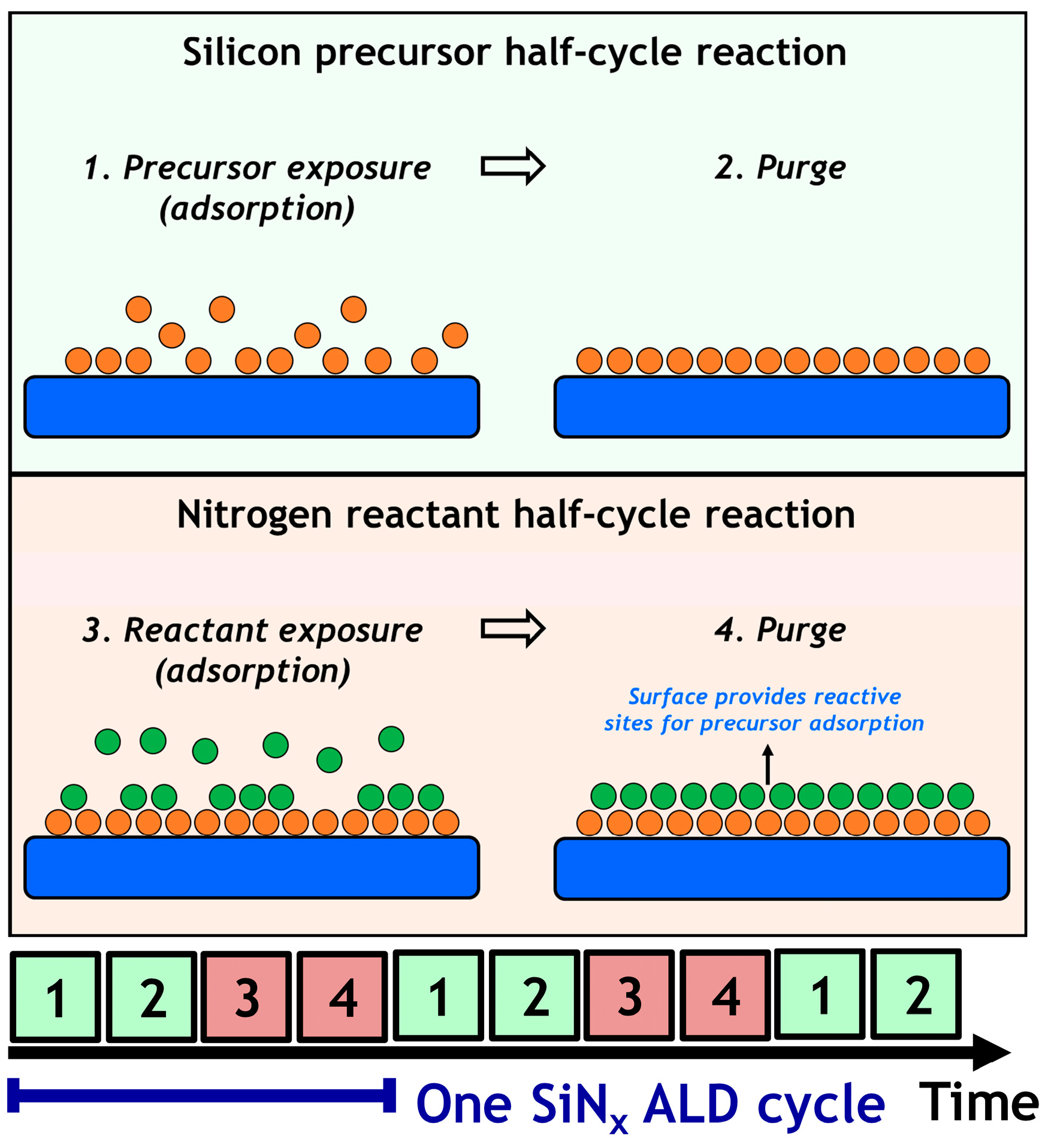


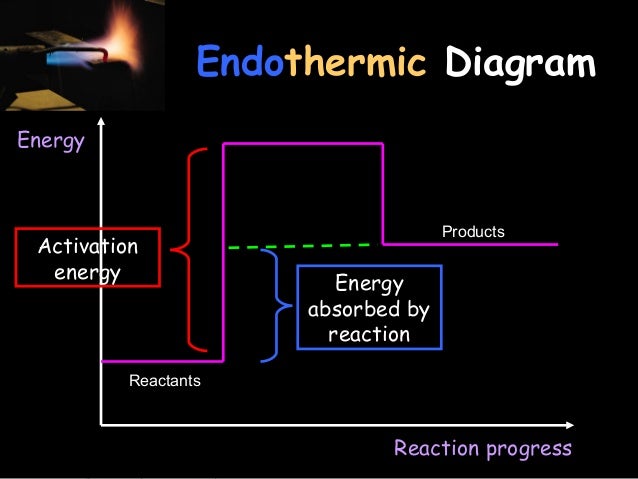
0 Response to "38 progress of reaction diagram"
Post a Comment