44 ch4 molecular orbital diagram
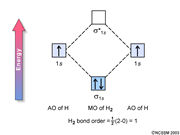
OCl2 Lewis Structure, Molecular Structure, Hybridization, Bond Angle ... We start with the two outer Chlorine atoms. This is done per the octet rule. The 4 remaining valence electrons act as lone pairs and attach themselves to Oxygen as shown in the figure. This is the final Lewis structure for OCl2. The Chlorine atoms have 8 valence electrons on each. The oxygen atom in the center also has 8 valence electrons. CHEM 123 Sapling Learning Chapter 11 Flashcards - Quizlet Construct the molecular orbital diagram for H+2 . A 1 s orbital from an H atom and a 1 s orbital from an H plus cation combine to form the molecular orbitals sigma 1 s and sigma 1 s star for the molecular H 2 plus. Sigma 1 s is lower in energy than the atomic orbitals and sigma 1 s star is higher in energy than the atomic orbitals.
techiescientist.com › ch4-lewis-structureCH4 Lewis Structure, Molecular Geometry, and Hybridization Jun 19, 2022 · Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
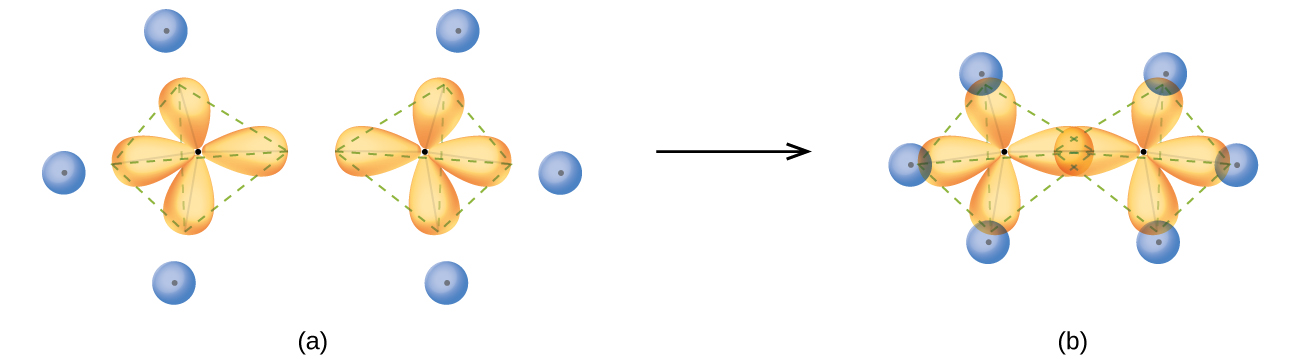
Ch4 molecular orbital diagram
Molecular Orbital Theory: Tutorial and Diagrams - Study.com Learn how to apply molecular orbital theory to determine the shapes of bonded orbitals, recognize molecular orbital diagrams, calculate bond order, and determine relative bond strength. Updated ... Formation of Molecular Orbitals (LCAO Method) | Important The term 2ψ 1 ψ 2 arises due to overlapping and fusion of atomic orbitals.. Thus, the probability of finding the electron is more in molecular orbital formed according to the equation I (∵ ψ b 2 > ψ 1 2 + ψ 2 2) and the new orbital is known as Bonding Molecular Orbital (BMO).The probability of finding an electron in Molecular orbital formed by LCAO according to equation II is less than ... James E. Brady The Molecular Nature of Matter (6th Edition) … James E. Brady The Molecular Nature of Matter (6th Edition) Copia. Eduardo Silva. Download Download PDF. Full PDF Package Download Full PDF Package. This Paper. A short summary of this paper. 15 Full PDFs related to this paper. Read Paper. Download Download PDF.
Ch4 molecular orbital diagram. Molecular Orbital Theory: Postulates, Configuration-Embibe Atomic orbitals: Molecular orbitals: Their electron cloud extends around the nucleus of a single atom, i.e., the atomic orbital is monocentric. Their electron cloud extends around all the nuclei of bonded atoms in the molecule, i.e., a molecular orbital is polycentric. CH4 Lewis Structure & Molecular Geometry - What's Insight CH4 Molecular Geometry Methane has tetrahedral molecular geometry with bond angles of 109.5° degrees. There are four covalent connections between hydrogen atoms and central carbon atoms in the Methane molecule. According to Valence shell electron pair repulsion (VSEPR) theory, the same kind of electron pairs repels each other. Viewing Molecular Orbitals - Avogadro Viewing Molecular Orbitals. This feature requires a "checkpoint" or "formatted checkpoint" from quantum chemistry codes. When the output file is opened, if a matching checkpoint file is found, it automatically opens the Orbitals toolbar. All potential molecular orbitals will have full status bars (you may need to scroll down ... Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
CF4 Tetrafluoride Lewis Structure, Molecular Structure, Hybridization ... CF4 comprises a Carbon atom surrounded by four Fluorine atoms. In its most stable state, the Carbon atom forms covalent atoms with the Fluorine atoms. There are no lone pairs. The hybridization of the CF4 is given by sp3. CF4 has a Tetrahedral molecular structure and shape with bond angles of 109.5°. About Priyanka Molecular Geometry Chart: Definition, Examples, and Study … Atoms have a nucleus and within this nucleus they have their own electrons rotating around. When orbitals are overlapped to create molecules via bonding, these types of orbitals are named molecular orbitals. Molecular orbital theory and valence bond theory both explain the properties of molecular and atomic orbitals. Orbitals can hold two ... What is the molecular geometry of h2s, clf3, brf5, clo2, ch4, h2co ... Because, according to VSEPR theory, molecular geometry considers only bond pairs or atoms, while electron geometry considers bonded atoms and lone pairs present on the central atom, CH4's molecular geometry and electron geometry are tetrahedral. The molecule H2 is a diatomic molecule with the chemical formula H2. Singlet oxygen - Wikipedia The 1 Δ g singlet state is 7882.4 cm −1 above the triplet 3 Σ − g ground state., which in other units corresponds to 94.29 kJ/mol or 0.9773 eV. The 1 Σ + g singlet is 13 120.9 cm −1 (157.0 kJ/mol or 1.6268 eV) above the ground state.. Radiative transitions between the three low-lying electronic states of oxygen are formally forbidden as electric dipole processes.
CHapter 11 Flashcards | Quizlet A molecular orbital is a region of space in a covalent species where electrons are likely to be found. The combination of two atomic orbitals always forms two molecular orbitals; the bonding molecular orbital, which is _____ in energy, and the antibonding molecular orbital, which is _____ in energy than the original atomic orbitals. quizlet.com › 552869460 › chem-123-sapling-learningCHEM 123 Sapling Learning Chapter 11 Flashcards - Quizlet Construct the molecular orbital diagram for H+2 . A 1 s orbital from an H atom and a 1 s orbital from an H plus cation combine to form the molecular orbitals sigma 1 s and sigma 1 s star for the molecular H 2 plus. Sigma 1 s is lower in energy than the atomic orbitals and sigma 1 s star is higher in energy than the atomic orbitals. PF3 lewis structure, Molecular geometry, Polar or nonpolar, Bond … Hence, the fluorine atom goes outside in the lewis diagram whereas the phosphorus atom fetched the seat of the central position. 3. Connect phosphorous and fluorine with a single bond ... “The exchange of electrons between an atomic orbital on one atom and an antibonding orbital on another atom is known as ... CH4 lewis structure, Molecular ... Molecular Orbital Theory MCQs With FREE PDF - LiveMCQs b) A molecular orbital is singly occupied. c) An example is oxygen molecule. d) Repelled by the magnetic field. Answer: Repelled by the magnetic field. 8. Combination of two atomic orbitals results in the formation of two molecular orbitals namely. a) one bonding and one non-bonding orbital. b) two bonding orbitals.
Molecular orbitals diagrams of [Co(NH3)6]3+ - SlideShare Molecular orbitals diagrams of [Co (NH3)6]3+. Nov. 21, 2021. • 0 likes • 1,757 views. Mithil Fal Desai. Download Now. Download to read offline. Description.
Orbitals And Molecular Representation Apr 06, 2022 뜀 Molecular Orbital diagram of CH4. The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals
8 - Drawing Molecular Orbital Diagrams — Flux Science The way these bonds are placed on any molecular orbital diagram is according to how the atomic orbitals that make the MOs mix. In that mixing, there are two factors to consider: (1) atomic symmetry and (2) mixing. Image via Chegg Symmetry As a bond occurs, a bond or internuclear axis- the line that connects the nuclei of two bonded atoms - forms.
Chemical Bonding and Molecular Structure Class 11 Chemistry Notes A molecular orbital that is formed by addition overlap (i.e., when the lobes of atomic orbitals overlap with the same sign) of two atomic orbitals is known as bonding molecular orbital. It is represented as ψ MO = ψ A + ψ B Its energy is lower than the atomic orbitals from which it is formed. It favours bonding.
Molecular orbitals diagrams of [Ti(H2O)6]3+ Molecular orbitals diagrams of [Ti(H2O)6]3+ 1. M. O. diagram for [Ti(H2O)6]3+ Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa
en.wikipedia.org › wiki › MethaneMethane - Wikipedia Methane (US: / ˈ m ɛ θ eɪ n / MEH-thayn, UK: / ˈ m iː θ eɪ n / MEE-thayn) is a chemical compound with the chemical formula CH 4 (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas.
Chemical Bonding and Molecular Structure Class 11 Notes ... - Learn CBSE A compound in which sp 3 hybridisation occurs is, (CH 4). The structures of NH 2 and H 2 0 molecules can also be explained with the help of sp 3 hybridisation. • Formation of Molecular Orbitals: Linear Combination of Atomic Orbitals (LCAO) The formation of molecular orbitals can be explained by the linear combination of atomic orbitals.
NO3 Lewis Structure, Molecular Geometry, and Hybridization 20/06/2022 · Nitrogen’s p orbital makes a bond with three terminal oxygen atoms. Molecular Geometry of NO3. As per VSEPR theory, you conclude that NO3 is sp2 hybridized. The model also states that the molecular geometry of the compound is trigonal planar with each orbital equidistant at 120 degrees (bond angle) shaped on a planar region.
MCQ of Chemical Bonding and Molecular Structure | Ch 4 | Chemistry MCQ of Chemical Bonding and Molecular Structure, Chapter 4, Chemistry, Class 11 Question 1: In a covalent bond formation, Transfer of electrons takes place Equal sharing of electrons between two atoms takes place Electrons are shared by one atom only Electrons are donated by one atom in shared by both atoms.
Molecular Orbital Theory - GeeksforGeeks The Molecular Orbital Theory is a chemical bonding theory developed at the turn of the twentieth century by F. R. Hund and R. S. Mulliken to explain the structure and properties of various molecules. The valence-bond theory failed to adequately explain how certain molecules, such as resonance-stabilized molecules, contain two or more equivalent ...
Answered: MO diagram (b | bartleby A: Molecular orbital diagram is the representation of molecular orbital of a compound in increasing… Q: draw the ressonace hybrid for these two structures A: So many resonating structure can be formed for both of the compound , so I am showing only resonance…
biologyjunction.com › molecular-geometry-chartMolecular Geometry Chart: Definition, Examples, and Study Guides Atoms have a nucleus and within this nucleus they have their own electrons rotating around. When orbitals are overlapped to create molecules via bonding, these types of orbitals are named molecular orbitals. Molecular orbital theory and valence bond theory both explain the properties of molecular and atomic orbitals. Orbitals can hold two ...
What is the Difference Between Sigma and Pi Molecular Orbitals Sigma molecular orbitals are types of hybrid orbitals that form from the overlapping of two atomic orbitals from head-to-head along the internuclear axis. Typically, the first covalent bond between two atoms is always a sigma bond. Overlapping of two atomic orbitals in the inter-nuclear axis forms a sigma covalent bond.
Draw The Molecular Orbital Energy Level Diagram Of O_2. Label The ... Label The Symmetry Of All Molecular Orbitals And Indicate From Which Atomic Orbitals They Are Obtained, Ii) Use The Diagram To Determine The Multiplicity Of O_2 (Singlet Or Triplet) In Its Ground Electronic State, Iii) Sketch The Shape (S) Of The Highest Occupied Molecular Orbital (S) Of O_2 And Indicate Apr 11 2022 05:51 AM Expert's Answer
CH4 Polarity - What's Insight CH4 Molecular Geometry. Methane's molecular geometry is tetrahedral, with bond angles of 109.5°. In the Methane molecule, four covalent bonds exist between hydrogen atoms and core carbon atoms. ... in the valence shell of carbon, one 2s orbital and three 2p orbitals combine to produce four sp3 hybrid orbitals of equal energy and shape. In ...
› questions-and-answers › moAnswered: MO diagram (b | bartleby A: Molecular orbital diagram is the representation of molecular orbital of a compound in increasing… Q: draw the ressonace hybrid for these two structures A: So many resonating structure can be formed for both of the compound , so I am showing only resonance…
Computational chemistry - Wikipedia Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of molecules, groups of molecules, and solids.It is essential because, apart from relatively recent results concerning the hydrogen …
CO2 Lewis Structure (2021 UPDATED) All You Need To Know In a Molecular Orbital Diagram, the 2s orbital of oxygen is nonbonding because of the high energy difference between carbon and oxygen atoms. ... CH4 + 2O2 ——-> CO2 + 2H2O. 3. This process uses combustion, where carbon-based fuels are produced through thermal decomposition. Calcium Carbonate goes through the heating process and forms ...


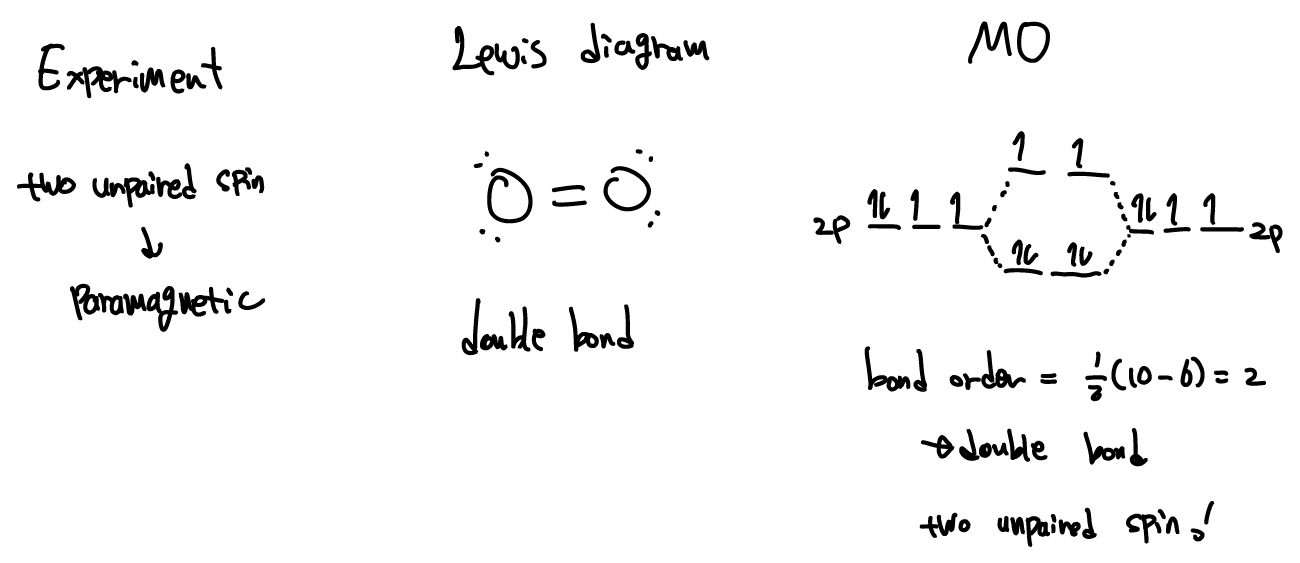





0 Response to "44 ch4 molecular orbital diagram"
Post a Comment