41 examine the following phase diagram and determine what phase exists at point d.
Phase Diagrams - Phases of Matter and Phase Transitions Point D is the point where all three phases meet. When the material is at this pressure and temperature, it can exist in all three phases. This point is called the triple point. The other point of interest is when the pressure and temperature are high enough to be unable to tell the difference between the gas and liquid phases. According to valence bond theory the triple bond in - Course Hero 98.Examine the following phase diagram and determine what phase exists at point F. SEE QUESTION 14 Image A) vapor + liquidB) vapor C) liquid D) solidE) supercritical fluid B ) vapor 99.Neon condenses due to A) dipole-dipole forces.B) London dispersion forces. C) hydrogen bonding. D) covalent bonding.E) intramolecular forces. B )
Ternary Phase Diagrams - Tulane University First determine all possible subsolidus assemblages by drawing the compositional triangles. These are also called 3 phase triangles, since a maximum of 3 phases can coexist in a ternary system below the solidus. To do this, examine each of the cotectics and determine what solid phases are in equilibrium along the curve.

Examine the following phase diagram and determine what phase exists at point d.
Phase Diagrams - Chemistry - University of Hawaiʻi Using the phase diagram for water, we can determine that the state of water at each temperature and pressure given are as follows: (a) solid; (b) liquid; (c) liquid; (d) gas; (e) solid; (f) gas. Check Your Learning What phase changes can water undergo as the temperature changes if the pressure is held at 0.3 kPa? If the pressure is held at 50 kPa? Phase Diagrams - an overview | ScienceDirect Topics Phase diagrams are core tools in solid physics and materials science. The phase diagrams of many alloys and some oxides are studied thoroughly and well known. However, there is limited data on the phase diagrams of nanoalloys. This is because this area of research is relatively new and drawing new phase diagrams is difficult. Lesson Plan: Phase Diagrams and Phase Equilibria Further, examine the phase diagrams of water and carbon dioxide, and discuss specific temperature and pressure conditions at which the different phases of these substances are stable/unstable. Use example 12.4.1 and exercise 12.4.2 included in the text to discuss phase transitions of water.
Examine the following phase diagram and determine what phase exists at point d.. 3 Examine the following phase diagram and determine what phase exists ... Examine the following phase diagram and determine what phase exists at point F. A) vapor + liquid B) vapor C) liquid D) solid E) supercritical fluid vapor The correct answer is letter B) vapor. 4. Neon condenses due to A) dipole-dipole forces. D) covalent bonding. B) London dispersion forces. E) intramolecular forces. C) hydrogen bonding. B ) 10.4 Phase Diagrams - Chemistry - opentextbc.ca Using the phase diagram for water, we can determine that the state of water at each temperature and pressure given are as follows: (a) solid; (b) liquid; (c) liquid; (d) gas; (e) solid; (f) gas. Check Your Learning What phase changes can water undergo as the temperature changes if the pressure is held at 0.3 kPa? If the pressure is held at 50 kPa? M11Q1: Features of Phase Diagrams - Chem 103/104 Resource Book Using the phase diagram for water, we can determine that the state of water at each temperature and pressure given are as follows: (a) solid; (b) liquid; (c) liquid; (d) gas; (e) solid; (f) gas. Check Your Learning What phase changes can water undergo as the temperature changes if the pressure is held at 0.3 kPa? If the pressure is held at 50 kPa? HQ 12 - Chapter 12 -Consider the following phase diagram ... - Course Hero Chapter 12-Consider the following phase diagram and identify the process occurring as one goes from point C to point D. Increasing temperature with a phase change from solid to vapor -Examine the following phase diagram and determine what phase exists at point F.
Chapter 10/11/12 Terms Flashcards | Quizlet Examine the following phase diagram and determine what phase exists at point F. SEE QUESTION 14 Image A) vapor + liquid B) vapor C) liquid D) solid E) supercritical fluid b Neon condenses due to A) dipole-dipole forces. B) London dispersion forces. C) hydrogen bonding. D) covalent bonding. E) intramolecular forces. c 57 Features of Phase Diagrams (M11Q1) - Unizin Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ... Solved 12) Examine the following phase diagram and identify - Chegg transcribed image text: 12) examine the following phase diagram and identify the feature represented by point a. 760 torr temperature a) melting point b) critical point c) triple point d) sublimation point e) boiling point 13) in hydrogen iodide are the most important intermolecular forces a) b) c) dipole-dipole forces london dispersion forces … Solved Examine the following phase diagram and determine | Chegg.com Transcribed image text: Examine the following phase diagram and determine what phase exists at point D. Pressure B. 760 torr А. C. D Temperature O liquid O supercritical fluid O gas and liquid gas O solid Previous questionNext question COMPANY About Chegg Chegg For Good College Marketing Corporate Development Investor Relations Jobs
13.5 Phase Changes - College Physics for AP® Courses - OpenStax There are well-defined regions on these graphs that correspond to various phases of matter, so PT PT size 12{ ital "PT"} {} graphs are called phase diagrams. Figure 13.28 shows the phase diagram for water. Using the graph, if you know the pressure and temperature you can determine the phase of water. The solid lines—boundaries between phases ... Phase Diagrams - an overview | ScienceDirect Topics Generalized phase diagrams have been proposed for both intra-lanthanide (Fig. 3 A [16]) and intra-actinide (Fig. 3 B [17]) alloy systems.These have proven to be quite useful in understanding the phase relationships in the two series of the elements and in predicting the existence of phases when experimental data are lacking, especially in the lanthanide series. PDF Chapter 9: Phase Diagrams - Florida International University Phase Diagrams: # and types of phases • Rule 1:If we know T and Co, then we know: --the # and types of phases present. • Examples: A(1100°C, 60): 1 phase: α B(1250°C, 35): 2 phases: L + α Adapted from Fig. 9.3(a), Callister 7e. Ternary Phase Diagram - an overview | ScienceDirect Topics A ternary phase diagram shows possible phases and their equilibrium according to the composition of a mixture of three components at constant temperature and pressure. Figure 4.23 shows a schematic of a ternary phase diagram. Single-phase regions are areas that originate from the vertex of the triangle and that are not enclosed by black curves. Two-phase regions are areas enclosed by black ...
Solved Examine the following phase diagram and determine - Chegg Transcribed image text: Examine the following phase diagram and determine what phase exists at point 760 som Temperature A) supercritical fluid B) liquid C) vapor+liquid D) vapor E) solid The phase diagram of a substance i s given below.
PHASE DIAGRAMS OF PURE SUBSTANCES - chemguide Suppose you have a pure substance at three different sets of conditions of temperature and pressure corresponding to 1, 2 and 3 in the next diagram. Under the set of conditions at 1 in the diagram, the substance would be a solid because it falls into that area of the phase diagram. At 2, it would be a liquid; and at 3, it would be a vapour (a gas).
160 questions with answers in PHASE DIAGRAMS - ResearchGate You will obtain a diagram with x axis : Temperature in K and y axis C activity (refered to Graphite). You can predict the equilibria between liquid, alloy (bcc, fcc) and carbides. See Example 1 ...
Phase Diagrams - Purdue University You can therefore test whether you have correctly labeled a phase diagram by drawing a line from left to right across the top of the diagram, which corresponds to an increase in the temperature of the system at constant pressure. When a solid is heated at constant pressure, it melts to form a liquid, which eventually boils to form a gas.
Solved Examine the following phase diagram and determine | Chegg.com See the answer Examine the following phase diagram and determine what phase exists at point C. 1. gas and liquid 2. gas 3. liquid 4. solid 5. supercritical fluid Show transcribed image text Expert Answer 100% (7 ratings) Transcribed image text: «D Temperature Previous question Next question
2 Component Phase Diagrams - Tulane University Eutectic point - the point on a phase diagram where the maximum number of allowable phases are in equilibrium. When this point is reached, the temperature must remain constant until one of the phases disappears. A eutectic is an invariant point. Peritectic point - The point on a phase diagram where a reaction takes place between a previously ...



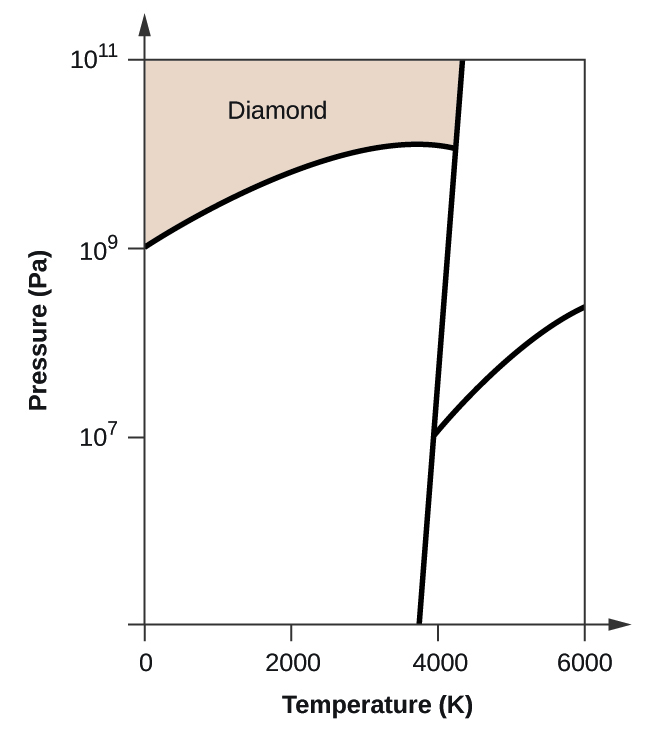
0 Response to "41 examine the following phase diagram and determine what phase exists at point d."
Post a Comment