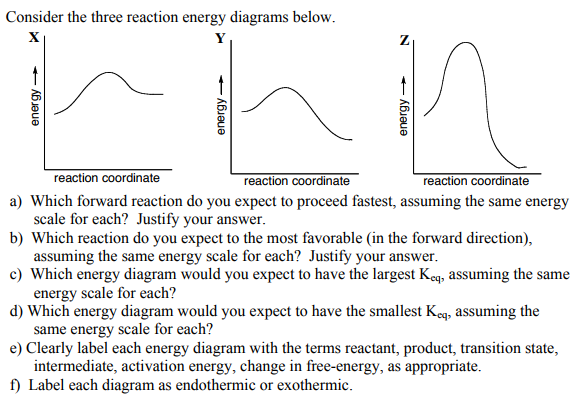
38 energy reaction coordinate diagram
Answered: B Potential energy Reaction Reaction… | bartleby Find answers to questions asked by students like you. The reaction below has Kp = 8.90 at 25°C. Br₂ (g) + Cl₂ (g) = 2 BrCl (g) If a sealed container…. An analytical chemist is titrating 220.1 mL of a 0.6700 M solution of benzoic acid (HC H,CO₂) with a…. Q: Answer the following questions based in the scheme below: Determine the ... PDF Energy Changes Accompany Each Elementary Step The essential components of a reaction coordinate diagram for a one-step reaction are shown above. Most important is the MEP on this diagram. The particular MEP on the above diagram might, for example, apply to the proton transfer from phenol to hydroxide. The reactants (a) are higher in energy than the products (c), so there is a favorable driving
Energy profile (chemistry) - Wikipedia Reaction coordinate diagrams are derived from the corresponding potential energy surface (PES), which are used in computational chemistry to model chemical reactions by relating the energy of a molecule (s) to its structure (within the Born-Oppenheimer approximation ).
Energy reaction coordinate diagram
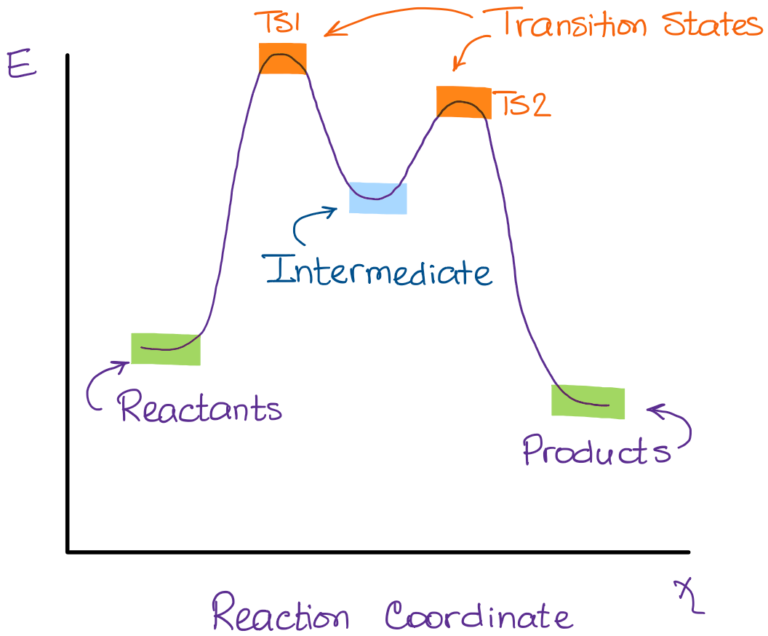
Reaction coordinate - Wikipedia In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. In molecular dynamics simulations, a reaction coordinate is called collective variable. Energy Diagrams, Transition States, and Reactive Intermediates ... - JoVE 2.11: Energy Diagrams, Transition States, and Intermediates Free-energy diagrams, or reaction coordinate diagrams, are graphs showing the energy changes that occur during a chemical reaction. The reaction coordinate represented on the horizontal axis shows how far the reaction has progressed structurally. Endothermic Reaction Coordinate Diagram - Wiring Diagrams In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other. Let's consider a general reaction where a reactant or set of reactants, A, or set of products, B. The diagram below is called a reaction coordinate diagram. B is at a lower total energy than A.
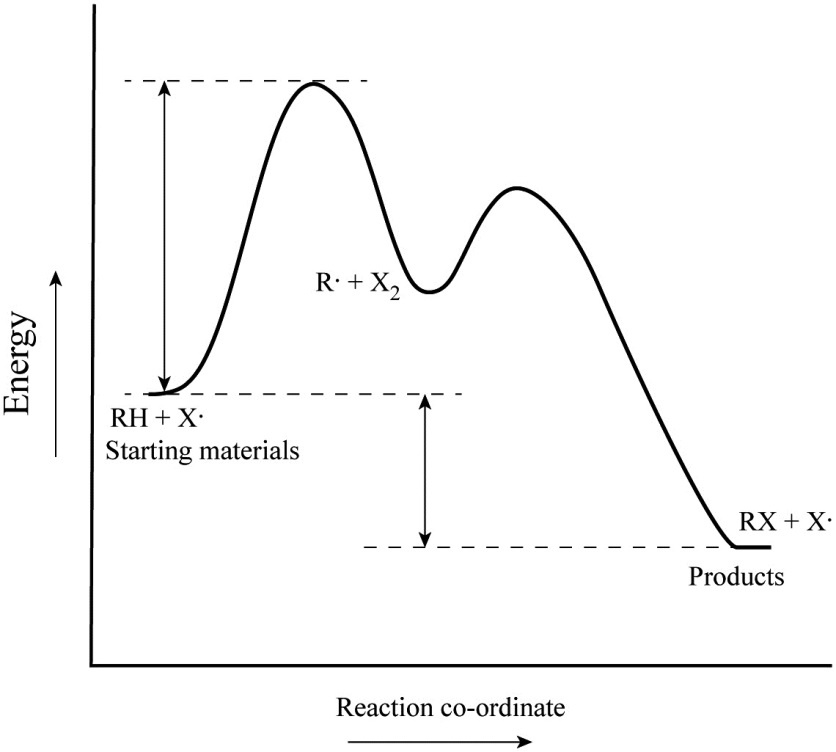
Energy reaction coordinate diagram. 6.6: Reaction Coordinate Diagrams - Chemistry LibreTexts In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this: Solved Label the following reaction coordinate diagram. - Chegg Label the following reaction coordinate diagram. Energy Reactant (s) Transition State Product (s) Activation Energy (forward) Transition State Activation Energy (forward) Energy Enthalpy of Enthalpy of Reaction Product (s) Reaction AHrxn Reactant (s) Reaction Coordinate Reaction Coordinate Reset Zoom Arrhenius Theory and Reaction Coordinates - Chemistry 302 A reaction coordinate is a path that links the reactant molecules and the products molecules. In many reactions, we can directly envision this coordinate as the length of a particular bond or bonds. In other cases, the reaction coordinate is used merely to represent some unknown coordinate. Energy/Reaction Coordinate Diagrams Thermodynamics, Kinetics A Reaction Coordinate (Energy) Diagram. Gibbs standard free energy change (DGo) Enthalphy (DHo): the heat given off or absorbed during a reaction Entropy (DSo): a measure of freedom of motion Thermodynamic Quantities DGo = DHo - TDSo DG,DH,DS,DE are state functions E = q + w H = qp If DSo is small, compared to DHo, then DGo ≈DHo. Entropy (ΔS)
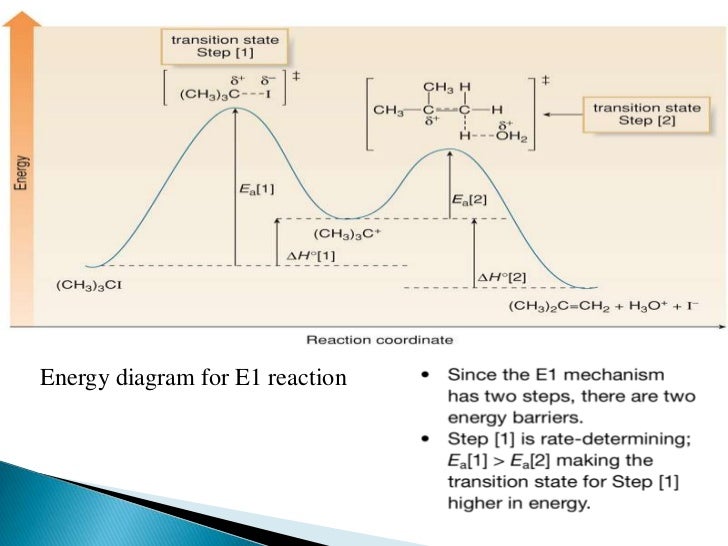
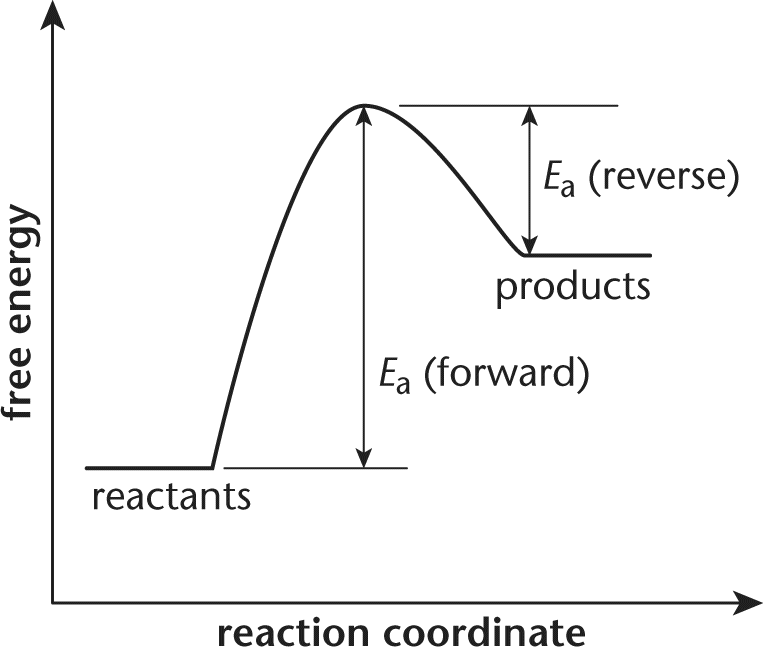
How can I draw activation energy in a diagram? | Socratic 1. Draw and label a pair of axes. Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4. PDF Energy/Reaction Coordinate Diagrams - chemconnections Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction Entropy (ΔSo): a measure of freedom of motion ΔGo = ΔHo - TΔSo ΔG,ΔH,ΔS, ΔE are state ... Reaction Coordinate Diagrams - College Chemistry The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants. Report an Error E1 Reaction Coordinate Energy Diagram - YouTube presents: E1 Reaction Coordinate Energy Diagram with step by step mechanism, transition states and intermediates📺Watch Next...
6. Reaction Coordinate Diagram - VIZISCIENCE® INTERACTIVE LABS The reaction coordinate diagram is represented below: Things to note. ΔH represents the difference between enthalpy of reactants and products. Ea1 and Ea2 represent the activation energy for step 1 and step 2 in the reaction. The step with the highest activation energy is the slowest step reaction. Sn1 Reaction Coordinate Diagram - schematron.org Figure 2: Reaction coordinate diagram for an SN1 reaction1. 1. Identify the . Figure 2 identifies these species in a reaction coordinate diagram like the one in the right-hand panel of Figure 1. Here the nucleophile is hydroxide ion. The SN1 Mechanism. 4. Determining Draw a reaction coordinate diagram that illustrates your equilibrium prediction. PDF Energy Diagrams I - chemconnections.org Energy Diagrams I Dr. Ron Rusay Fall 2008 A Reaction Coordinate Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction Entropy (ΔSo): a measure of freedom of motion ΔGo = ΔHo - TΔSo ΔG,ΔH,ΔS, ΔE are state functions If ΔSo is small compared to ΔHo ... Solved Label the reactants, products, and energy changes in - Chegg Label the reactants, products, and energy changes in the reaction coordinate diagram below. Define the reaction as endothermic or exothermic. Is this reaction endothermic or exothermic? Reactants Activat energy Products Overall energy change Exothermic Reaction pathway Endothermic
Quiz & Worksheet - Energy Reaction Coordinate Diagrams | Study.com 1. Which point on the following reaction coordinate diagram represents the activation energy? Point A Point B C (red-dashed line) D (green-dashed line) 2. Which point represents delta E (the change...
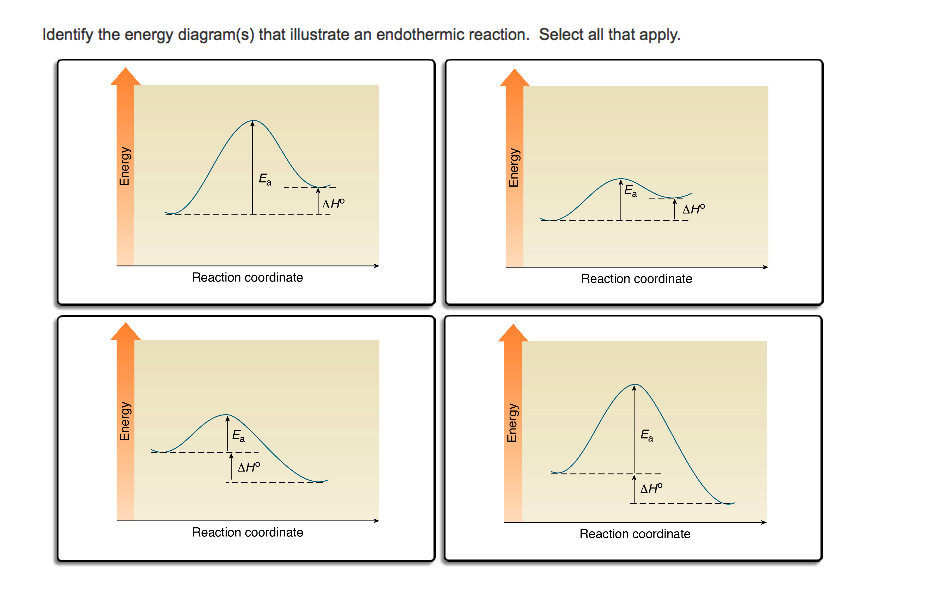
Reaction Coordinate Diagram Endothermic Vs Exothermic A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change there are many ENDOTHERMIC reactions such as photosynthesis that occur.! An Exothermic (Exergonic) Reaction! 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O! A reaction with ∆H°0 is endothermic.
PDF Thermodynamics vs Kinetics - Columbia University A general Reaction Coordinate Diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the figure below. In the figure below, the Activation Energy, Ea is that critical minimum energy in a chemical reaction required by reactants to be converted into products. the quantities, Ea;
Practice: Reaction Coordinate Energy Diagrams - YouTube In this video, Dr. Norris goes over practice problems in interpreting reaction energy coordinate diagrams.
Energy Diagram Catalyzed Vs Uncatalyzed Reaction Reactants Products + Energy. Draw a reaction coordinate diagram for this reaction as above but add the activation energy, E a, for the catalyzed reaction on the appropriate curve in this diagram and label it. This is a bit more subtle since .Types of catalysts (article) | Kinetics | Khan AcademySection The Rate of a Reaction ...
Sketch a qualitative reaction energy diagram for a | Chegg.com Sketch a qualitative reaction energy diagram for a chemical reaction with and without a catalyst. Assume the uncatalyzed reaction is endothermic. Note: Because the sketches are only qualitative, the energies in them don't have to be exact. They only have to have the right relationship to each other. For example, if one energy is less than ...
Reaction Coordinate Diagrams The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction (heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
Chapter 5: Unit 10. Energy Diagram - gsusurveychemistry.org Energy Diagram A potential energy diagram illustrates the energy changes that occurs during a chemical reaction. Energy is plotted along vertical axis and reaction coordinate is plotted along horizontal axis. According to collision theory, reactant molecules collide during chemical reaction.
Analyzing Energy With a Reaction Coordinate Diagram - Study.com All reactions require energy, from the simplest - such as boiling water to turn liquid water into gaseous water - to the complex, such as electrochemical reactions. We can see what is happening to...
What is a reaction coordinate diagram? The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. ... start subscript, a, c, t, end subscript and Δ H ΔH ΔH for any reaction from its energy diagram. Hereof, what is a reaction profile? A reaction profile is a diagram showing the change in chemical potential ...
Endothermic Reaction Coordinate Diagram - Wiring Diagrams In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other. Let's consider a general reaction where a reactant or set of reactants, A, or set of products, B. The diagram below is called a reaction coordinate diagram. B is at a lower total energy than A.
Energy Diagrams, Transition States, and Reactive Intermediates ... - JoVE 2.11: Energy Diagrams, Transition States, and Intermediates Free-energy diagrams, or reaction coordinate diagrams, are graphs showing the energy changes that occur during a chemical reaction. The reaction coordinate represented on the horizontal axis shows how far the reaction has progressed structurally.
Reaction coordinate - Wikipedia In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. In molecular dynamics simulations, a reaction coordinate is called collective variable.

.gif?revision=1&size=bestfit&width=960&height=720)


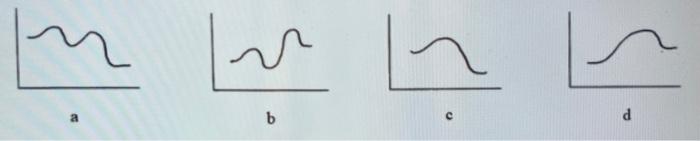
0 Response to "38 energy reaction coordinate diagram"
Post a Comment