38 construct the octahedral crystal-field splitting diagram for the metal in each species.
Construct the octahedral crystal-field splitting diagram for the metal ... Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ Mn (H2O)6^2+ 23,749 results, page 8 PHYSICS A thin 2.82 m long copper rod in a uniform magnetic field has a mass of 44.2 g. When the rod carries a current of 0.238 A, it floats in the magnetic field. The acceleration of gravity is 9.81 m/s2 . 10.png - Construct the octahedral crystal-field splitting diagram for ... 10.png - Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H, O) Fe (CN) * - Mn (H, O) + 1 1 - 1 1 1 1 1 1 Answer 10.png - Construct the octahedral crystal-field splitting... School University of Phoenix Course Title CHEM 4051 Uploaded By GeneralFishMaster387 Pages 1 This preview shows page 1 out of 1 page.
Solved Construct the octahedral crystal-field splitting | Chegg.com This problem has been solved! See the answer. See the answer See the answer done loading. Construct the octahedral crystal-field splitting diagram for the metal in each species. V ( H 2 O ) 3 + 6 V (H2O)63+ Co ( CN ) 3 − 6 Co (CN)63− Mn ( H 2 O ) 2 + 6 Mn (H2O)62+ Construct the octahedral crystal-field splitting diagram for the metal in ...
Construct the octahedral crystal-field splitting diagram for the metal in each species.
PDF | Cell (Biology) | Biochemistry - Scribd Each class of polymeric biomolecule has a different set of subunit types.[1] For example, a protein is a polymer whose subunits are selected from a set of 20 or more amino acids. Biochemistry studies the chemical properties of important biological molecules, like proteins, and in particular the chemistry of enzyme-catalyzed reactions. The biochemistry of cell metabolism and the … Construct The Octahedral Crystal-field Splitting Diagram For The Metal ... Show transcribed image text Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+% (20). Lecture 9 - Crystal field theory for octahedral, way as the octahedral crystal field stabilization energy. in the square plane move a little closer to the metal. Breaking the Scaling Relationship Limit: From Single-Atom to Dual … 17.05.2022 · ConspectusRecent decades have witnessed the rapid development of catalytic science, especially after Taylor and Armstrong proposed the notion of the “active site” in 1925. By optimizing reaction paths and reducing the activation energies of reactions, catalysts appear in more than 90% of chemical production reactions, involving homogeneous catalysis, …
Construct the octahedral crystal-field splitting diagram for the metal in each species.. The tetrahedral crystal field - Big Chemical Encyclopedia The splitting pattern of the 4f orbitals in a tetrahedral crystal field can be deduced in a similar manner. If we adopt the coordinate system shown in Fig. 8.11.4, we can obtain the splitting pattern shown in the same figure. As expected the 1 3 3 pattern for the tetrahedral crystal field is just the reverse of the 3 3 1 pattern of the octahedral field. ... Chemically exfoliated inorganic nanosheets for nanoelectronics 02.06.2022 · Therefore, reviewing them together not only provides an efficient understanding of the synthesis, integration, and possible applications of each class of nanosheets but also emphasizes the current common issues to be solved for further development of high-performance devices using these exfoliated nanosheets. 2D metal oxides, TMDCs, and MXenes cover a … Crystal Field Theory - an overview | ScienceDirect Topics In aqueous solution, first-row transition metal M 2+ are surrounded by an inner solvation shell of six water molecules, which leads to an octahedral, or nearly octahedral, M 2+ (H 2 O) 6 species [85]. Crystal field theory states that the resulting field splits the degenerate atomic 3d orbitals into molecular e g and t 2g orbitals. Construct the octahedral crystal-field splitting diagram for Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+ Option 1 Low Cost Option Download this past answer in few clicks 2.85 USD PURCHASE SOLUTION Option 2 Custom new solution created by our subject matter experts GET A QUOTE rated 5 stars Purchased 3 times Completion Status 100%
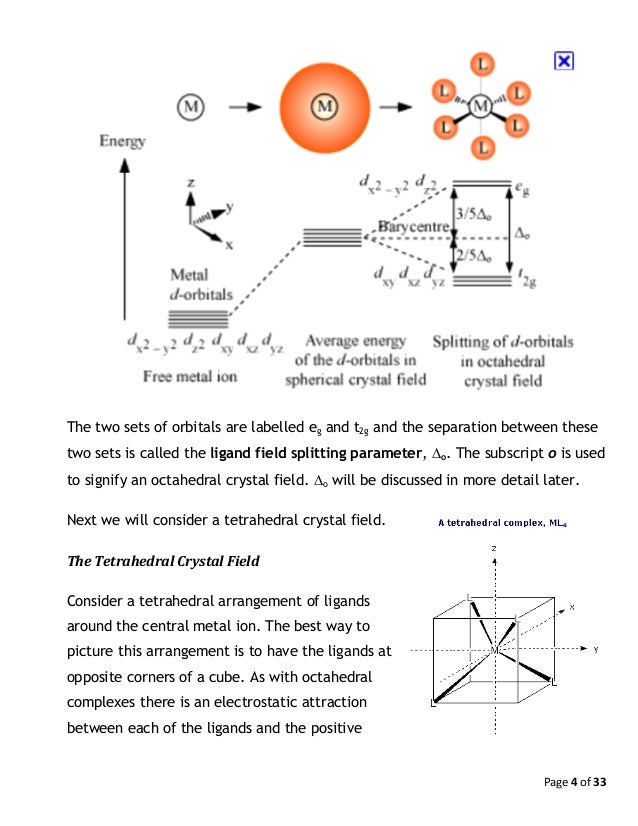
Construct the octahedral crystal-field splitting diagram for the metal ... For each metal complex, give the coordination number for the metal species. Na [Ag (CN)2 [Cd (en)Br2 mathematics, algebra The diagram shows a scale drawing of a lacrosse field. The diagram is 5 1/2 inches long and 3 inches wide. If 1 inch represents 20 yards, what is the area of the field. A. 6,000 square yards B. 6,600 square yards C. 1,650 square Crystal Field Theory (Theory) : Inorganic Chemistry Virtual Lab ... The octahedral arrangement of six ligands surrounding the central metal ion is as shown in the figure. In an octahedral complex, the metal ion is at the centre and the ligands are at the six corners. In the figure, the directions x, y and z point to the three adjacent corners of the octahedran. Crystal Field Theory - Purdue University The difference between the energies of the t 2g and e g orbitals in an octahedral complex is represented by the symbol o.This splitting of the energy of the d orbitals is not trivial; o for the Ti(H 2 O) 6 3+ ion, for example, is 242 kJ/mol. . The magnitude of the splitting of the t 2g and e g orbitals changes from one octahedral complex to another. It depends on the identity of the metal ion ... Answered: Construct the octahedral crystal-field… | bartleby Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63− Mn (H2O)62+ Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution star_border Students who've seen this question also like: General Chemistry - Standalone book (MindTap Course List)
PDF D-orbital splitting diagrams - University of California, Berkeley D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. Trigonal bipyramidal 4. Square pyramidal d z2x2-y d xy d yzxz 5. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d ... inorganic 3 Flashcards | Quizlet how to construct the octahedral crystal-field splitting diagram for the metal in each species. start by determining how many d electrons each metal species possesses. Make sure that degenerate orbitals obey Hund\'s rule of maximum multiplicity. ... The absorption causes a d-to-d transition between t2g and eg orbitals in the octahedral splitting ... Crystal field theory - Wikipedia The crystal field splitting energy for tetrahedral metal complexes (four ligands) is referred to as Δ tet, and is roughly equal to 4/9Δ oct (for the same metal and same ligands). Therefore, the energy required to pair two electrons is typically higher than the energy required for placing electrons in the higher energy orbitals. Access Denied - LiveJournal Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.
Freestanding Metal–Organic Frameworks and Their Derivatives: An ... Metal–organic frameworks (MOFs) have recently emerged as ideal electrode materials and precursors for electrochemical energy storage and conversion (EESC) owing to their large specific surface areas, highly tunable porosities, abundant active sites, and diversified choices of metal nodes and organic linkers. Both MOF-based and MOF-derived materials in powder form have …
OneClass: Match the appropriate octahedral crystal-field splitting ... Get the detailed answer: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state ... In an octahedral crystal field, the d electrons on a metal ion occupy the eg set of orbitals before they occupy the t2g set of orbitals. (B) Diamagnetic metal ions cannot have an odd ...
[Solved] Construct the octahedral crystal-field splitting diagram for ... Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H, 0)6 Co (CN)6 Fect Answer Bank 1 1... Show more Please help me with this question, thanks. Chemistry Science Inorganic Chemistry CHEM 1200 Answer & Explanation Solved by verified expert
Construct The Octahedral Crystal-field Splitting Diagram Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in.
Inorganic Chemistry 4th edition, Catherine Housecroft Inorganic Chemistry 4th edition, Catherine Housecroft
PDF Crystal Field Splitting in an Octahedral Field - IIT Kanpur Distribution of Electrons in an Octahedral Complex d1 d2 d3 Strong field Weak field Strong field W eak field Strong field Weak field 1 2 Net energy decrease is called crystal field stabilization energy (CFSE) Ford1, CFSE = 1 × 0.4 = 0.4 Δ, CFSE oo For d2, CFSE = 2 × 0.4 = 0.8 Δ o For d3, CFSE = 3 × 0.4 = 1.2 Δ o
Construct The Octahedral Crystal-field Splitting Diagram For The Metal ... Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ Mn (H2O)6^2+. Nov 14, · Basically, the question is referring to the compound K3 [Fe (C2O4)3]. It asks what is the electron configuration in this comound, I got it to be d5. Fe in the compound is Fe (III) so 23 electrons -> d5.
Characterization techniques for nanoparticles: comparison and ... The presence of a solid solution type cubic structure in which cation sites were randomly occupied was observed. 133 Finally, for the characterization of surface species and substrate–surface interactions on metal NPs, the groups of Pruski and Emsley have shown that dynamic nuclear polarization surface enhanced NMR can be a very useful tool for the further increase of the …




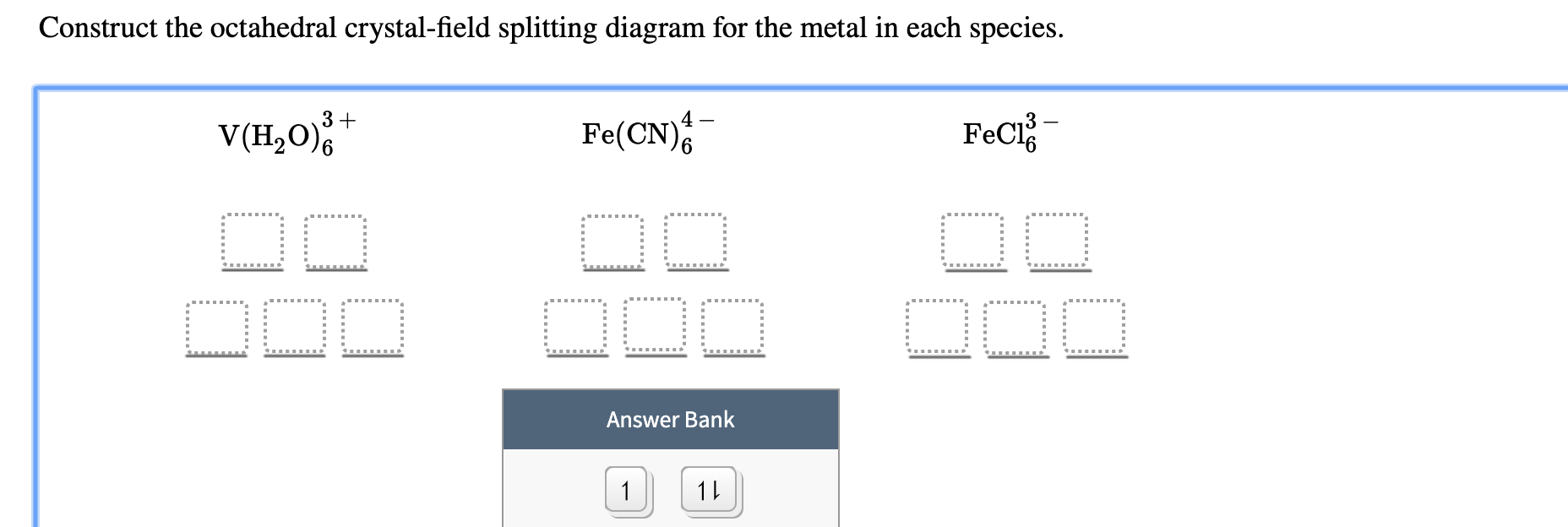

0 Response to "38 construct the octahedral crystal-field splitting diagram for the metal in each species."
Post a Comment