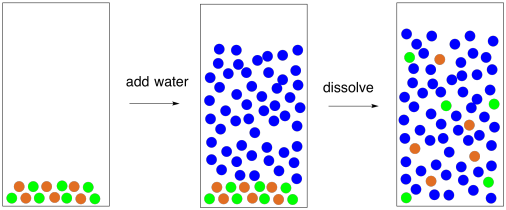
40 diagram of table salt dissolved in water
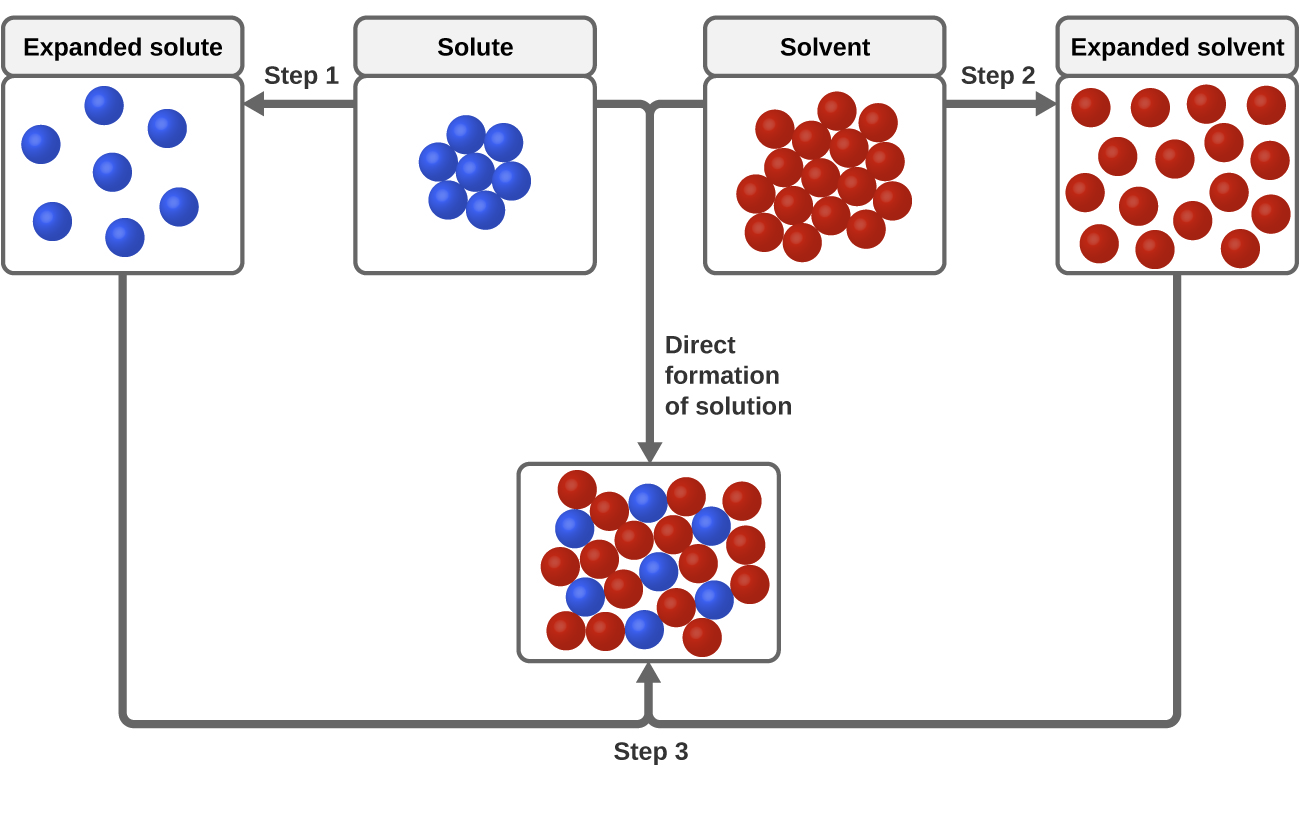
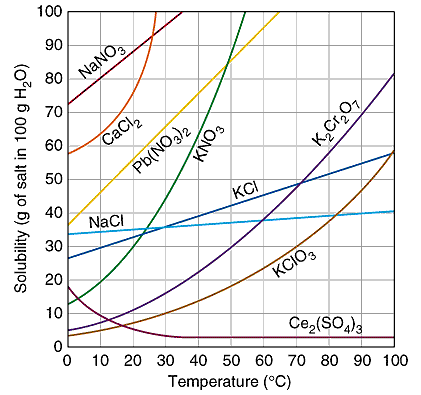
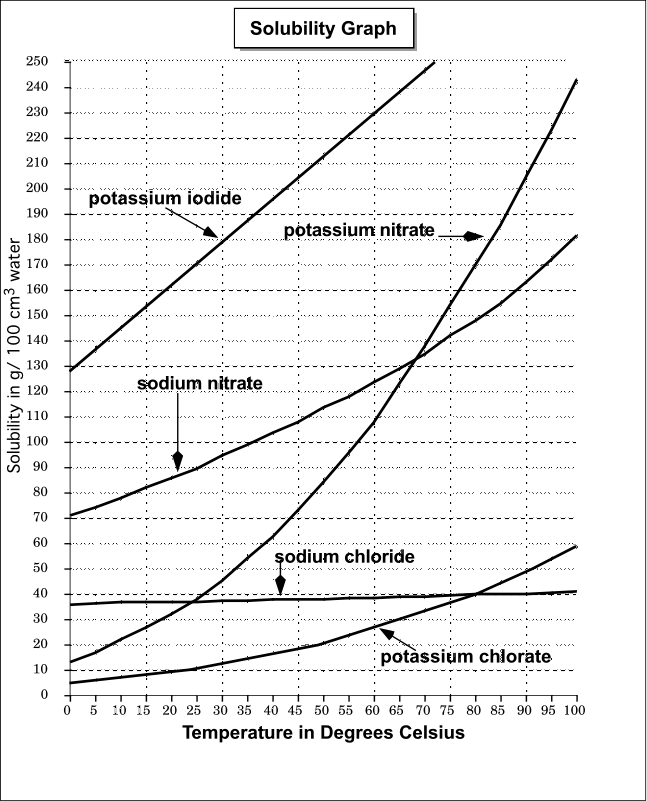
This post will go over details of relative humidity, why a salt test works, how it's so easy you never need buy a calibration kit, and finally how this all ties in to Boveda packs. #What is Relative Humidity and why is it important? Relative Humidity is the vapor pressure of the water vapor in a given space relative to the maximum vapor pressure for that same space. Another way to put it is that it's the amount of water vapor in a space compared to the maximum amount of water vapor that can f... 1. Use Microsoft Excel to construct a data table containing columns labeled with vial number, temperature (°C), mass of KNO 3, mass of water, and solubility (g salt /100 g solvent) for all six trials. Include a separate entry for the unknown solution's identification number, characteristic temperature, and concentration. 2.
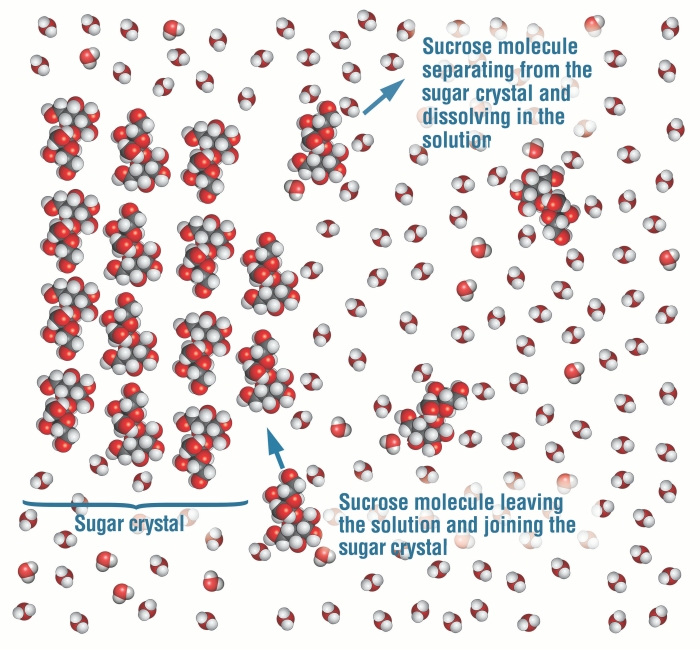
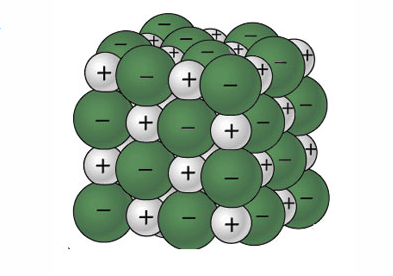
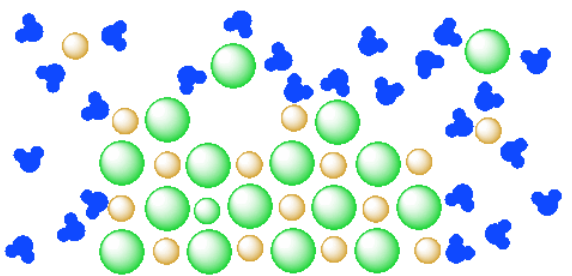
Table salt is made of the ionic compound sodium chloride, which consists of the chemical elements sodium and chlorine.You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; the more salt you add, the longer this takes, and it may require some shaking or stirring to bring about.

Diagram of table salt dissolved in water
If you mix two substances and the result is a homogeneous mixture, you are dealing with a solution. In the case of table salt mixed with water, Na and Cl atoms, initially bonded together in the form of a crystal, are dissolved by molecules of water. Water is a solvent. The reasons are electrostatic in nature. The cohesion of atoms and molecules derive from electrostatic links between particles ... Artificial lighting means any source of light that does not come from the sun. Natural lighting is defined light that comes from the sun or other stars. Traditionally, artificial light comes from torches, oil lamps, or magical lamps. Generally, none of these involve any living organisms, and rely on combustion. However, there are alternatives to this method of classification, and they rely on something else: living organisms. In order to describe them, we’re going to have a new, ad-hoc category:... FIGURE: Piper diagram of water chemistry in bedrock wells in Ogden Valley, Weber County, Utah. The location of symbols in the triangles shows the relative proportion of cations and anions in samples, whereas the size of the symbols is proportional to the amount of total dissolved solids (TDS) in the samples.
Diagram of table salt dissolved in water. How does water dissolve salts? When table salt, sodium chloride, dissolves in water, it dissociates into its respective cations and anions, Na+ and Cl-. 12.13 Calculate the density of FeO, given that it has the rock salt crystal structure. Solution We are asked to calculate the theoretical density of FeO. This density may be computed using Equation (12.1) as ! = n " (AFe+ AO) VCNA Since the crystal structure is rock salt, n' = 4 formula units per unit cell. Using the ionic radii for Fe2+ and O2-from Table 12.3, the unit cell volume is computed ... we can easily draw a diagram of sodium chloride dissolved in water. Let's just do some quick review, though. Sodium chloride is in a C l. And remember, sodium has a positive charge because it has one extra proton in the nucleus than electrons in the outer cloud. And chloride has one extra electrons in the outer plowed compared to the neutrons, protons that are in the nucleus with you and ... Answer (1 of 6): The table salt quite quickly dissociated into ions, with Na+ as the cation and Cl- as the anion. Two oppositely charged ions of the same magnitude… a solvation shell forms around each ion, in which the Na+ cation will be surrounded by the water molecules with the negative dipole ...
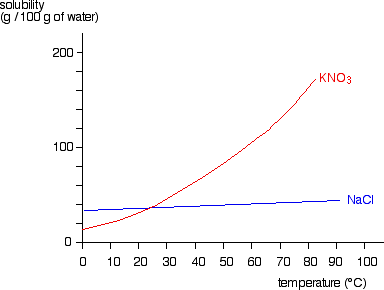
This page looks at the phase diagram for mixtures of salt and water - how the diagram is built up, and how to interpret it. It includes a brief discussion of solubility curves. Important: This page is only really designed to be an introduction to the topic suitable for courses for 16 -18 year olds, such as UK A level chemistry. Students will make a 2-D model of a salt crystal and use water molecule cut-outs to show how water dissolves salt. After seeing an animation of water dissolving salt, students will compare how well water and alcohol dissolve salt. They will relate their observations to the structure of salt, water, and alcohol on the molecular level. Objective It was their favorite prank. Isa Jones and Halshaa Vess were mini-celebrities at the University. Two Lords of War, from both species, both teaching history at a humble university many light-years away from their home. What had led them there was the source of unending rumors; they were political exiles, outlaws, scouts for eventual invasion, their "backstory" got more and more elaborate with each retelling. But when Isa and Halshaa both found out they were from The Deep, an idea formed betwe... The particular mixture of salt and water (containing 23.3% salt) which freezes at this temperature is known as a eutectic mixture. Using Phase Diagrams The phase diagram is used to find out what happens if you cool salt solution of a particular concentration.
Diagram shown represents rules obtained in a study of suspension containing both broken and whole cells. ... A mixture of sand and table salt can be separated by filtration because the substances in the mixture differ in ... Two grams of potassium chloride are completely dissolved in a sample of water in a beaker. This solution is classified as ... hi guys imma just type the summary of things we need to know for chem o lvl's tmr (pls add on in the comments if i miss out something so i can edit this again) in hoping to help out others while also helping me to revise bcos typing notes is wayy faster than writing :) also this wld not be super precise bcos im just typing in the summary if not i'm literally typing a whole textbook here, but i'll try my best to put in all the infos that is important \*totally not last minute\* **kinetic partic... This list combines my favorite entries from [these](https://www.reddit.com/r/d100/comments/7ykf1b/lets_build_100_magic_weather_effects/) [two](https://www.reddit.com/r/d100/comments/7quk2l/lets_build_100_magical_storms/) weather threads. I modified some entries from their original sources. d100| Uh oh, the magic barometer is reading low. The forecast for the upcoming weather is...|Credit ---------|----------|---------- 1 | Daggerfall: It rains daggers dealing 1d4 damage / round people are out u... Sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible ...
This list was abandoned and whoever originally posted it went and got their account deleted. Let's do it a favor and cast true resurrection! 1. An old but seemingly undamaged piece of abstract art containing a pattern designed to crash the viewer's brain. 2. A small box containing an AI brain, with no apparent means of interacting with the world other than a small speaker and an adapting USB like system. When picked up, it will immediately try to convince the PCs to load it into a more capable...
When a soluble ionic compound is placed in water, the solid is converted to the product of the dissolution reaction|the solid vanishes, converting to dissolved ions in solution. 3 Use the solubility rules to determine if the salt is soluble or insoluble, and enter that information in the table.
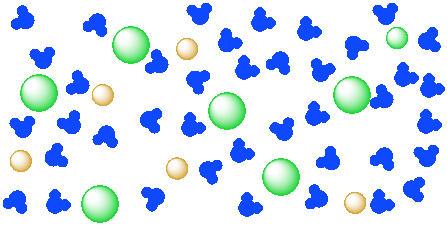
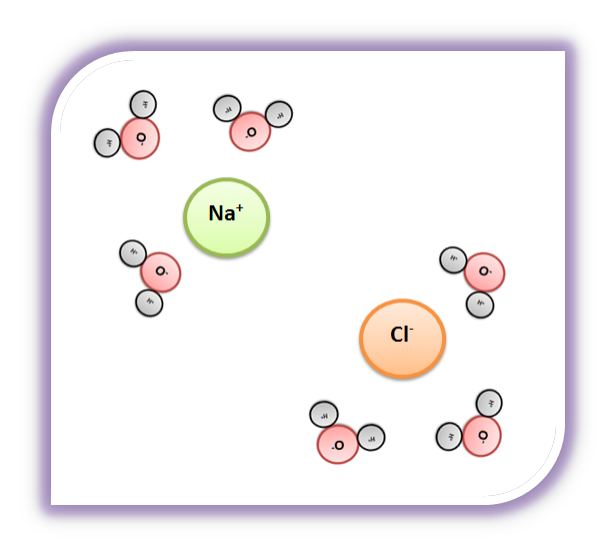
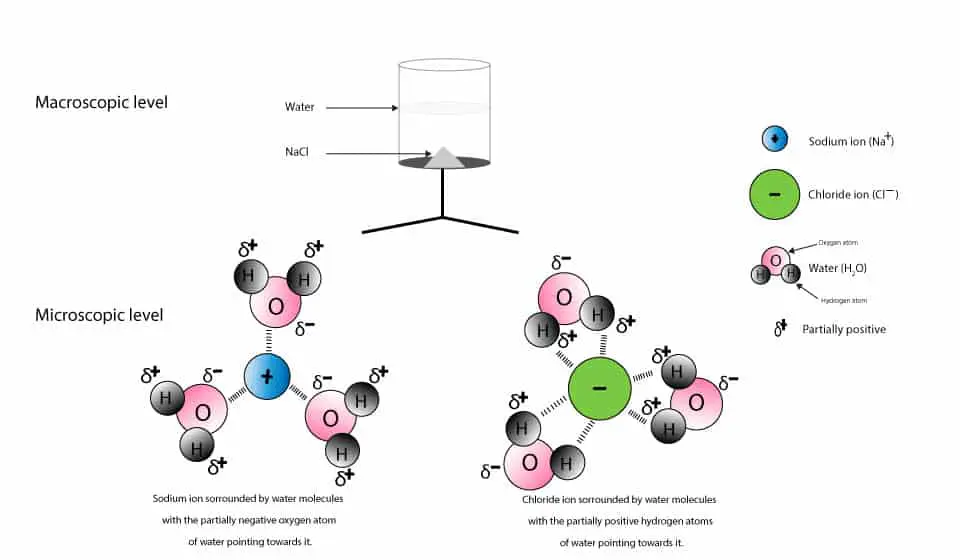
What happens when table salt dissolves in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a ...
Ordinary table salt is called sodium chloride. It is considered a substance because it has a uniform and definite composition. All samples of sodium chloride are chemically identical. Water is also a pure substance. Salt easily dissolves in water, but salt water cannot be classified as a substance because its composition can vary.
Water dissolves salt by dissociating the ions in salt from each other. Because water is a polar molecule, each of its ends holds a slight positive or negative electrical charge. These ends attract the positive and negative ions in salt and pull them apart from each other. The polarity of water comes from the differences in electronegativity in ...
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
In a solution of table salt and water, the salt is the solute and the water is the solvent. In the cells of your body, substances such as calcium ions and sugar are solutes, and water is the solvent. Water is the most common and important solvent, but other substances can also be solvents. For example, if you have ever used an oil-based paint
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Answer (1 of 2): If the salt,is really dissolved, it is a solution. Your teacher may want You to say that it is an aqueous solution of sodium chloride. You could also call it a homogeneous mixture. The salt is the solute and the water is the solvent. The chemical compound in table salt is sodium ...
When you dissolve table salt (sodium chloride, also known as NaCl) in water, are you producing a chemical change or a physical change? Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change.A physical change, on the other hand, results in a change of the material's appearance, but no new chemical products result.
14. Salt A and salt B were each dissolved in separate beakers of water at 21°C. The temperature of the salt A solution decreased, and the temperature of the salt B solution increased. Based on these results, which conclusion is correct? 1) The water gained energy from both salt A and salt B. 2) The water lost energy to both salt A and salt B.
Choose a salt. There are many different salts available, and they all have different properties.The amount of Epsom salt (MgSO 4) that you can dissolve into a given amount of water at a given temperature will differ from the amount of table salt (NaCl) that you can dissolve into the same water.. If you are trying to understand the dissolution process in general, you should stick with using ...
A mixture of crystals of salt and sugar is added to water and stirred until all solids have dissolved. Which statement best describes the resulting mixture? (1) The mixture is homogeneous and can be separated by filtration. (2) The mixture is homogeneous and cannot be separated by filtration.
Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na +) and chloride (Cl -) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three steps we previously discussed.
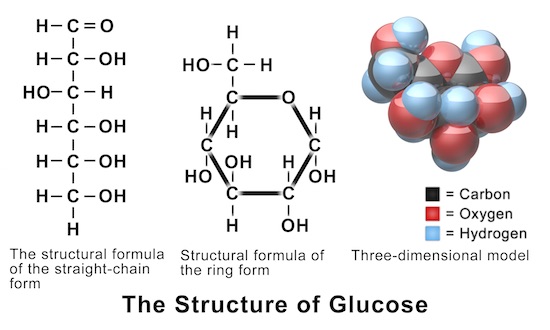
Find step-by-step Biology solutions and your answer to the following textbook question: Draw a diagram of table salt (NaCl) dissolved in water..1 answer · Top answer: $\textbf{\color{#c34632}Explanation:-}$ $\Rightarrow$ Water molecules ionize NaCl molecules and convert them into positive sodium ions and negative ...
Hello. Thank you for reading. I am struggling through an Organic Chemistry class at night while working two jobs. It is the last prerequisite before I can apply to go back to school for what I want to. I prepared this lab write-up a couple weeks ahead of time and after completing it last night I am struggling to write the conclusion. I will copy/paste the lab report (typed before transcribing into lab book) here. Everything is my words until "conclusion" where I typed the professors instructions...
Imagine, if you will, a crackling fire. Sticks blackening, a glowing red and orange heat. Flickering flames rising up. Smoke fills your nostrils. Dark, crisp, intense. Carried on its ashy wing’s hints of flavors of wood, ash, coal and cinder. You can smell the wood, the heat. You see the light bathing you, hear the snapping of coals, feel its warmth on your skin. There is a power to fire. Fire lights our nights and transforms our world. But, only in its power to cook, is fires raw destruction tr...
38 diagram of salt dissolved in water Written By Robert T. Arbuckle Saturday, December 4, 2021 Add Comment Edit Salt s dissolve in water to produce an anion and a cation. These ions make up the basis of conductivity in water.Conductivity is a measure of water 's capability to pass electrical flow. This ability is directly related to the concentration of ions in the water 1.
Electrolysis of water can be achieved in a simple hands-on project, where electricity from a battery is passed through a cup of water (in practice a saltwater solution or other electrolyte will need to be used otherwise no result will be observed). Electrolysis of an aqueous solution of table salt (NaCl, or sodium
C reacting potassium with water D using petrol in a motor car engine 15 The diagrams show some pieces of laboratory equipment. 3 thermometer 60 30 45 15 2 stop-clock 1 balance Which equipment is needed to find out whether dissolving salt in water is an endothermic process? A 1 only B 1 and 3 C 2 and 3 D 3 only
You could easily dissolve about 360 g of table salt in a liter of water, but the solubility of calcium carbonate is only about 0.01 grams per liter. That's partly due to the fact that the ions in sodium chloride, Na + and Cl-, have lower charges than the ions in calcium carbonate, Ca 2+ and CO 3 2- .
Seriously guys... we need to talk about this. I see more and more posts regarding how soil is superior, how better it is, how "organic" it is, how much more "natural" it is then all these "synthetic chemical" bottled nutrients... [https://en.wikipedia.org/wiki/Soil#Composition](https://en.wikipedia.org/wiki/Soil#Composition) " A typical soil is about **50% solids (45% mineral and 5% organic matter)**, and 50% voids (or pores) of which half is occupied by water and half by gas. " ...
FIGURE: Piper diagram of water chemistry in bedrock wells in Ogden Valley, Weber County, Utah. The location of symbols in the triangles shows the relative proportion of cations and anions in samples, whereas the size of the symbols is proportional to the amount of total dissolved solids (TDS) in the samples.
Artificial lighting means any source of light that does not come from the sun. Natural lighting is defined light that comes from the sun or other stars. Traditionally, artificial light comes from torches, oil lamps, or magical lamps. Generally, none of these involve any living organisms, and rely on combustion. However, there are alternatives to this method of classification, and they rely on something else: living organisms. In order to describe them, we’re going to have a new, ad-hoc category:...
If you mix two substances and the result is a homogeneous mixture, you are dealing with a solution. In the case of table salt mixed with water, Na and Cl atoms, initially bonded together in the form of a crystal, are dissolved by molecules of water. Water is a solvent. The reasons are electrostatic in nature. The cohesion of atoms and molecules derive from electrostatic links between particles ...










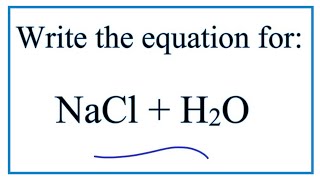


0 Response to "40 diagram of table salt dissolved in water"
Post a Comment