39 diagram of exothermic reaction
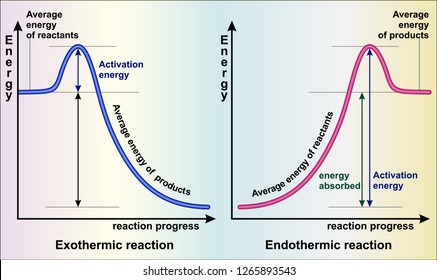
For an exothermic reaction, the enthalpy change is always negative. An energy level diagram for an endothermic reaction. In an endothermic reaction, the products are at a … This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
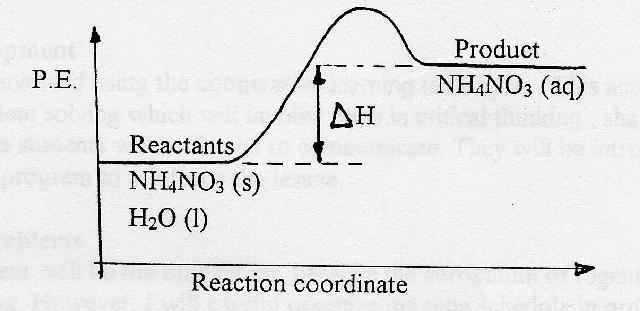
Diagram for Endothermic Reaction. Burning methane was an exothermic reaction, now how do we draw the energy diagram for an endothermic reaction? An example of this is when we dissolve ammonium ...

Diagram of exothermic reaction
An exothermic graph is one where potential energy is on the y-axis, the reaction pathway is on the x-axis and as the reaction progresses potential energy drops indicating the generation of thermal ... The energy level diagram helps to provide further understanding of the propagation process. The 1 st step in propagation is endothermic, while the energy absorbed can be offset by the 2 nd exothermic step. Therefore the overall propagation is exothermic process and the products are in lower energy level the than reactants. Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn , as well as DG 1 * and DG 2 * for the first and second activation energies, respectively.
Diagram of exothermic reaction. Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy. An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.". Based on the above definition, let's pick a few examples from our daily lives and categorize them as endothermic or exothermic. Start studying Exothermic and Endothermic Reactions. Learn vocabulary, terms, and more with flashcards, games, and other study tools. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2)
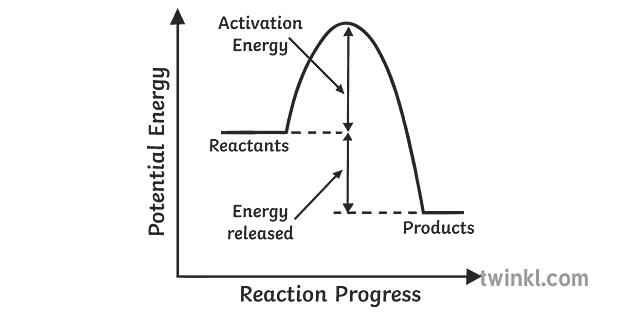
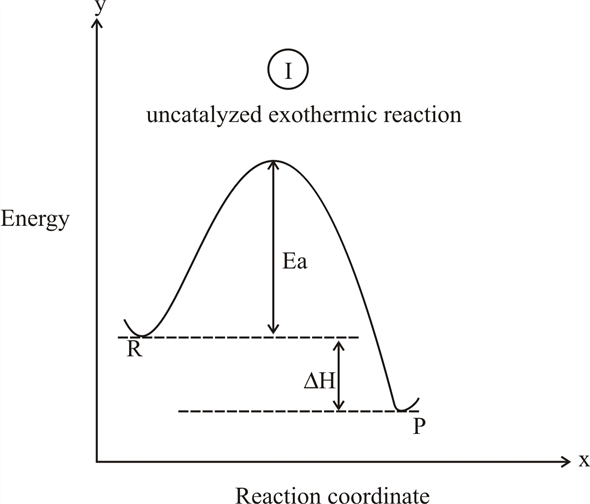
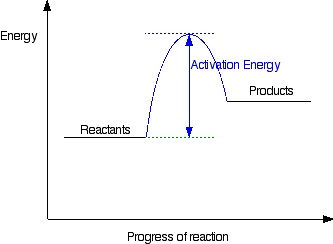
A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed. Shows whether a reaction is exothermic. Figure shows the energy level diagram for the reaction between methane and oxygen. The reaction shown by the second diagram is more exothermic. There is a greater difference in energy between the reactants and products. The green arrow is longer. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. An exothermic reaction is a chemical reaction that releases heat and has a negative enthalpy (-ΔH) and positive entropy (+ΔS).. These reactions are energetically favorable and often occur spontaneously, but sometimes you need a little extra energy to get them started.
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a … An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction Exothermic and Endothermic Reactions - Energy Level DiagramForm 5 Chemistry Chapter 4 ThermochemistryThis video is created by http://www.onlinetuition.com.my... Energy Profile for Exothermic Reactions. The synthesis of ammonia gas (NH 3 (g)) from nitrogen gas (N 2 (g)) and hydrogen gas (H 2 (g)) is an exothermic reaction. 92.4 kJ mol -1 (of N 2 (g)) is released. Energy (heat) is a product of the reaction: N 2 (g) + 3H 2 (g) → 2NH 3 (g) + 92.4 kJ mol -1. In order for energy to be conserved during the ...
Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ...
Energy Diagrams Both endothermic and exothermic reactions can be shown on energy diagrams. A way to tell if a diagram is endothermic or exothermic reaction is to look at the start and end of the graph. If the end part of the graph is more than the start, it is an endothermic reaction meaning it absorbed energy.
An exothermic reaction is the release of thermal energy (-ΔH) as it flows out of the system. Thermal energy is negative because energy is being released and the initial potential energy of reactants is more than the energy released from products. Let's explore the exothermic reactions occurring around us: 1.
In an exothermic reaction, is enthalpy change positive or negative? negative 8. In an endothermic reaction, is enthalpy change positive or negative? positive 9. When hydrochloric acid reacts with ammonium hydroxide in a beaker, the temperature goes up. HCl + NH 4 O H → NH 4 C l + H 2 O ΔH = –53.4kJ/mol Complete the energy profile diagram and state whether …
The chemical, exothermic reaction that is fire. Take a look at the following diagram, called the "Fire Triangle" Oxygen, heat, and fuel are frequently referred to as the "fire triangle." Add in the fourth element, the chemical reaction, and you actually have a fire "tetrahedron." The important thing to remember is: take any of these four things away, and you will not have a fire or the fire ...
01.12.2020 · Applications of exothermic and endothermic reactions in everyday life . Application of exothermic and endothermic reactions: The principle of exothermic and endothermic reactions is applied in instant cold packs and hot packs which are used to treat sports injuries. Instant cold packs have separate compartments of water and solid ammonium nitrate placed in …
An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative.
Exothermic Reactions is the flow of the net transfer of heat energy during the reaction is from the medium into its surroundings. In exothermic reactions, the reactants always possess more energy than the products and hence are less stable. For this reason, the exothermic reactions require very less amount of activation energy to initiate the reaction.
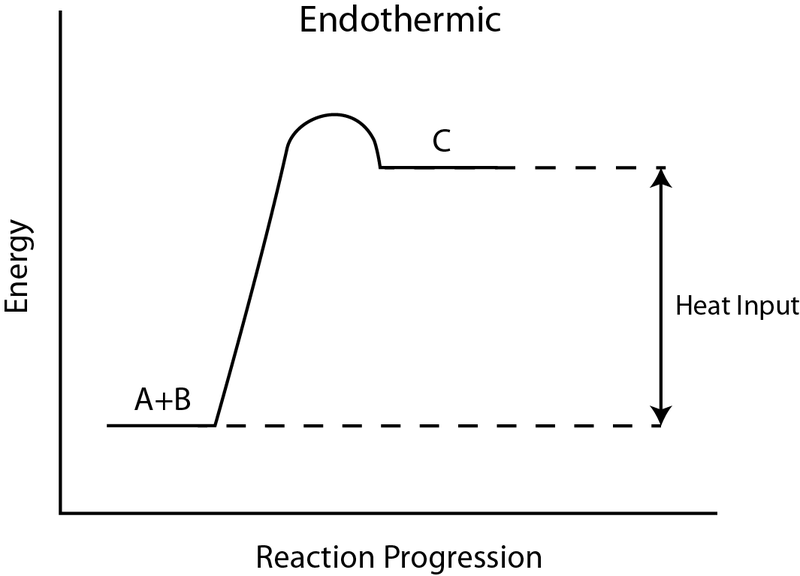
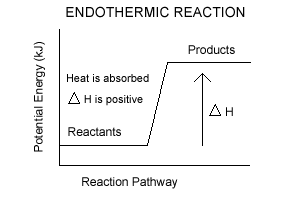
Endothermic and Exothermic Reactions. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Endothermic reactions require energy, so energy is a reactant. Heat flows from the surroundings to the system (reaction mixture) and the enthalpy of the system increases (ΔH is positive).
Exothermic reactions. The reactions that produce heat to the surrounding causing the increase in its temperature . Properties. The heat transfers from the system to the surrounding which leads to decrease the temperature of the system and increase the temperature of the surrounding .. ΔH° has a negative sign because heat content of the products is lower than that of the reactants .
The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
Exothermic Diagram. Energy released in bond making. Activation Energy. Energy used in bond. breaking. Exothermic - More energy is released when the products where formed than energy was used to break bonds in the reactants. Therefore, a net release of energy.
The enthalpy diagram for exothermic and endothermic reactions is shown below. Explanation : Endothermic reaction : It is defined as the chemical reaction in which the energy is absorbed from the surrounding. In the endothermic reaction, the energy of reactant are less than the energy of product.
Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
You can start with a generic potential energy diagram for an exothermic reaction.. A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed.. So, the activation energy is the minimum amount of energy required for a reaction to take place.
Chemical Reaction: A transformation ... shown in the following diagram. Here, the (R)-reactant gives the configurationally inverted (S)-product, and (S)-reactant produces (R)-product. The (R) and (S) notations for configuration are described in a later section of this text. (ii) Different rates of reaction, as in the base-induced elimination of cis & trans-4-tert-butylcyclohexyl bromide ...
It does not matter whether the reaction is an exothermic or an endothermic energy change (see the pair of reaction profile diagrams below). Higher temperature molecules in gases and liquids have a greater average kinetic energy and so a greater proportion of them will then have the required activation energy to react on collision.
History. The process was invented by Carl Friedrich Claus, a German chemist working in England.A British patent was issued to him in 1883. The process was later significantly modified by IG Farben.. Claus was born in Kassel in the German State of Hesse in 1827, and studied chemistry in Marburg before he emigrated to England in 1852. He died in London in 1900.
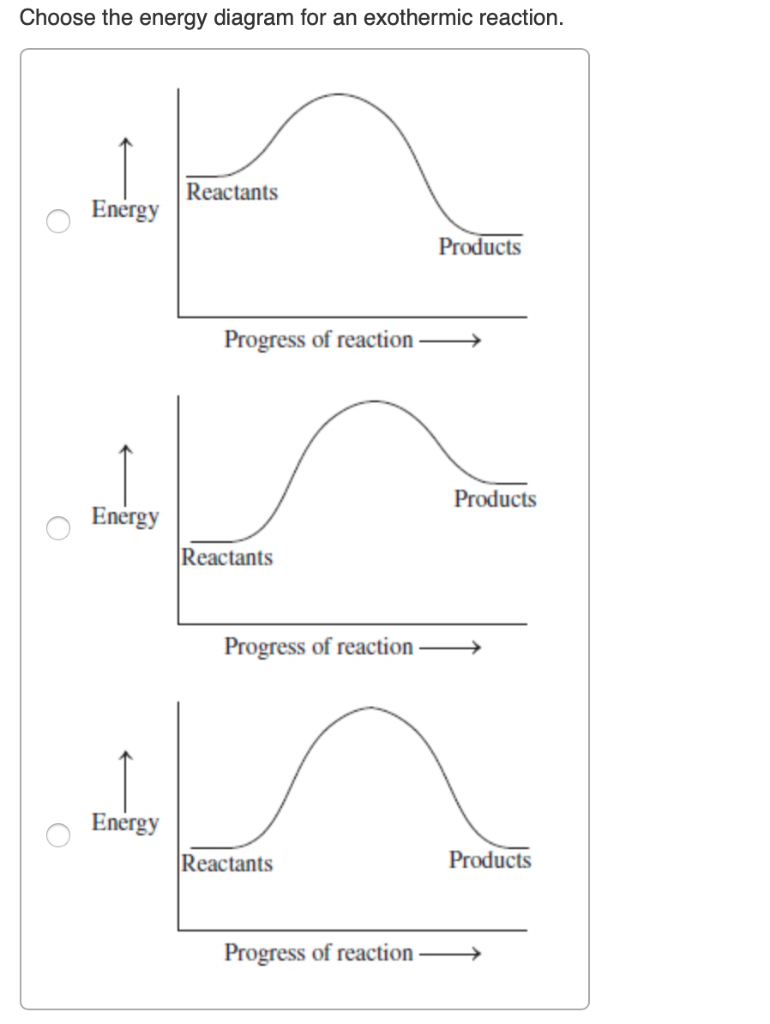
Which potential energy diagram represents an exothermic reaction? Potential Energy Potential Energy non Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential energy diagram ...
01.04.2020 · Progress of Reaction Energy Common oxidation exothermic processes are the combustion of fuels and the oxidation of carbohydrates such as glucose in respiration. 1.4 Energetics Definition: Enthalpy change is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. N Goalby chemrevise.org 2 …
Diagram of endothermic and exothermic reactions. Learn with flashcards, games, and more — for free.
Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn , as well as DG 1 * and DG 2 * for the first and second activation energies, respectively.
The energy level diagram helps to provide further understanding of the propagation process. The 1 st step in propagation is endothermic, while the energy absorbed can be offset by the 2 nd exothermic step. Therefore the overall propagation is exothermic process and the products are in lower energy level the than reactants.
An exothermic graph is one where potential energy is on the y-axis, the reaction pathway is on the x-axis and as the reaction progresses potential energy drops indicating the generation of thermal ...

















0 Response to "39 diagram of exothermic reaction"
Post a Comment