38 construct the orbital diagram for ni
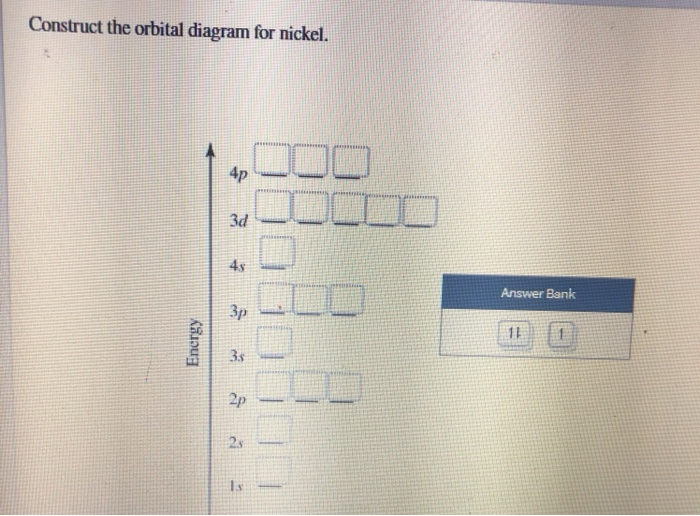
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Transcribed Image Textfrom this Question. Construct the orbital diagram for nickel 1 11 1 4p 1 11 14 1 3d 1 4s Answer Bank 1 1 1 3p 1 1 1L 3s 1 1 1L 2p 1 2s 1s Energy Construct the orbital diagram of the F ion Зр Answer Bank 3s 1 2p 2.s 1s Energy.
Solution for 23. Complete the atomic orbital diagram for Nickel (Ni) and answer the following questions (assume a ground state electron configuration): 1s ...
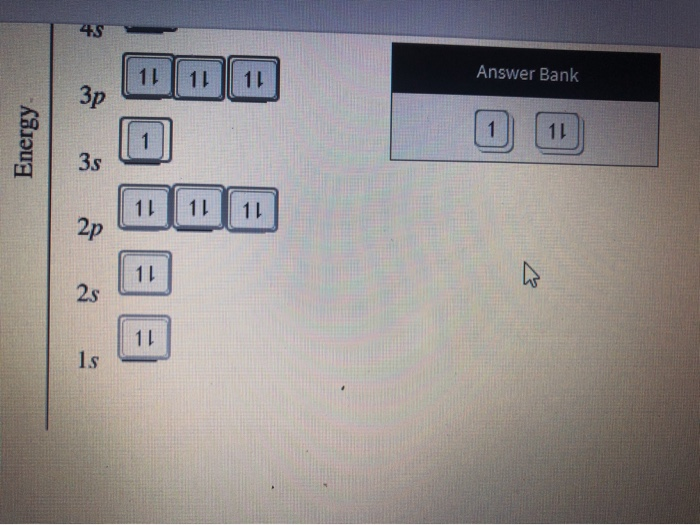
Construct the orbital diagram for ni
2. Molecular orbital theory: This is the best model to explain the bonding within the CO ligand as well as in metal carbonyl complexes. There are total three molecular diagrams for carbonyl ligand which were proposed from time to time. Though, all three molecular orbital (MO) diagrams are able to explain the nature of metal-4 (ii) Complete the diagram to show the splitting of the d orbital energy levels in an octahedral complex ion. energy (iii) On the axes below, sketch the shapes of one d orbital from the lower energy level and one d orbital from the higher energy level. y lower energy level z x y z x higher energy level [4] Construct the orbital diagram for ni. Construct the orbital diagram for ni. These are simplified diagrams of how electrons are arranged within the orbitals for a. The metal is produced by heating the ore in a blast furnace which replaces the sulfur with oxygen. Draw the orbital diagram for ion co 2.
Construct the orbital diagram for ni. So the atomic orbital diagram is simply those orbitals in that order of energy. Note that the #1s# orbitals are significantly lower in energy than the #2s# orbitals. For the homonuclear diatomic #"O"_2# , we simply have two copies of this atomic orbital diagram far apart at first. orbital diagram and that would force us to fill in the bonding sigma MO and the anti-bonding sigma-star MO. What we gain in the bonding sigma MO, we lose in the anti-bonding sigma-star MO. There is no advantage for two helium atoms to join together in a molecule, and so they remain as isolated atoms (note Question: Construct the orbital diagram for Ni. This problem has been solved! See the answer ... construct construct the orbital diagram for ni molecular orbital diagram a molecular orbital diagram or mo diagram is a qualitative descriptive tool. image file c7ta k f8 tif. electron configurations and orbital diagrams key electron configurations and orbital diagrams key draw orbital diagrams for the following elements 1 phosphorus.
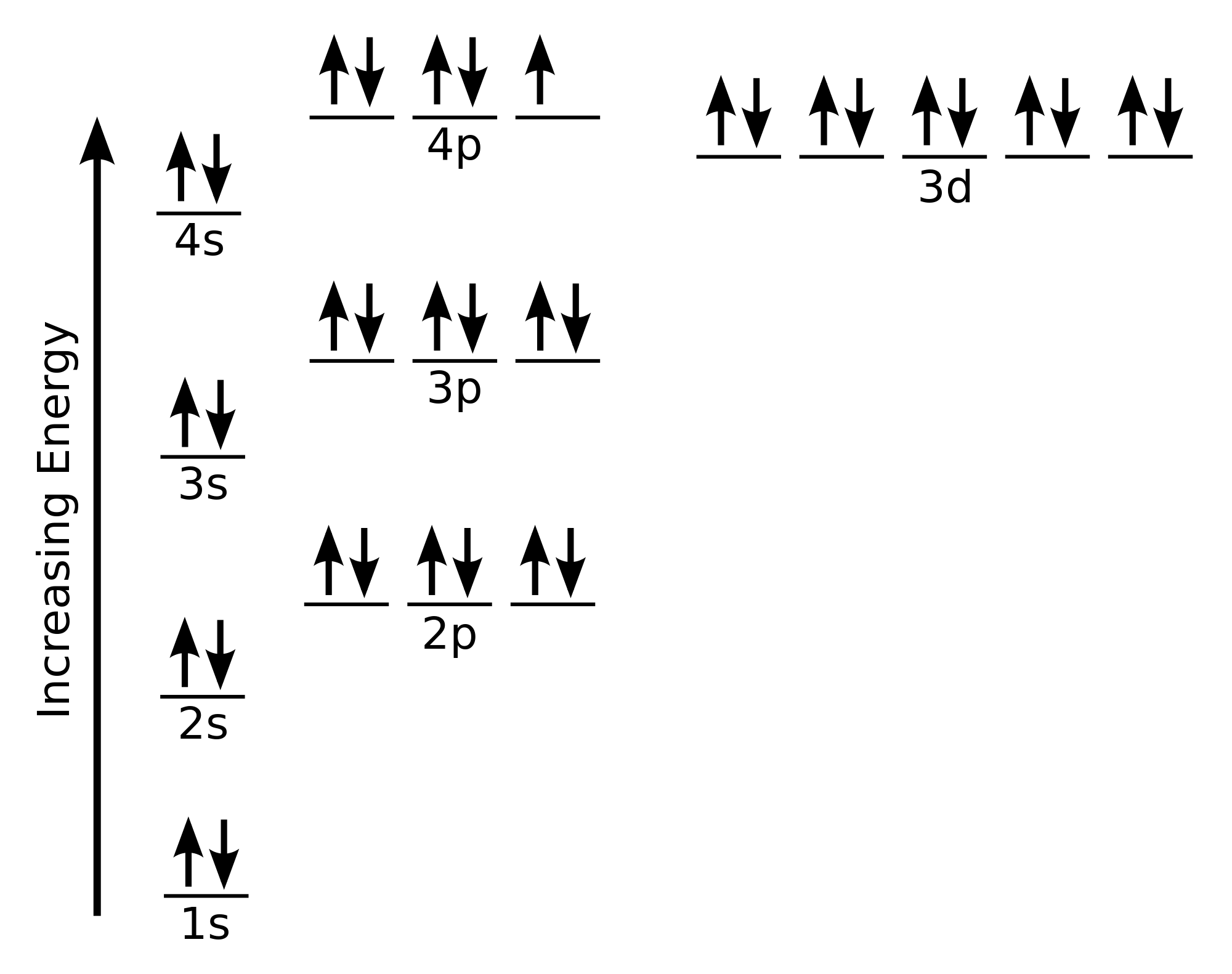
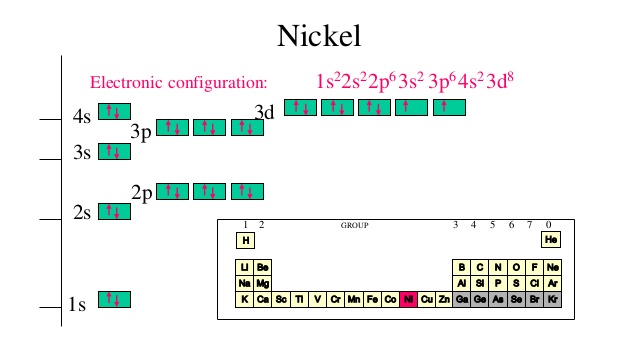
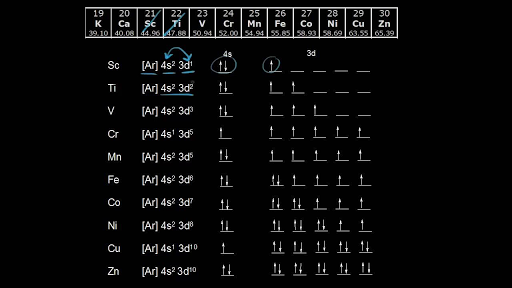
Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev... An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s. The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d shell, where the last two are up ... Construct The Orbital Diagram For Ni. 03.06.2019 03.06.2019. 76 Plymouth Duster Dome Light Wiring Diagram ... 03.06.2019 03.06.2019. 2007 Suzuki Forenza Wiring Diagram For Keyless Entry. 03.06.2019 03.06.2019. Rochester Quadrajet Carburetor Vacuum Diagram. 03.06.2019 03.06.2019. Tls2-gd2 Wiring Diagram ... Vanadium Orbital Diagram; 92 Cadillac ...
First start filling the electrons with lowest energy orbital and each orbital is singly occupied with one electron. And only two electrons with opposite spin ...1 answer · Top answer: Concepts and reason The concept used to solve this problem is based on orbital diagrams. Orbital diagrams are pictorial representation of total number ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Answer to Construct the orbital diagram for Ni. Start by adding the appropriate subshells. For example, carbon is in the 2p block.1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2.
Draw an orbital diagram, write a full electron configuration and core configuration for nickel. check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here. Want to see this answer and more? Experts are waiting 24/7 to provide step-by-step solutions in as fast as 30 minutes!*
For the orbital diagram, we simply need to draw each orbital and fill them up with the correct number of electrons. Take note that we need to ...Aug 27, 20181 answer · Top answer: We’re being asked to construct the orbital diagram for Ni. For that, we first need to determine the electron configuration of Ni.Recall that for a ...
Draw a molecular orbital diagram of ${N_2}$ or ${O_2}$ with magnetic behavior and bond order. Answer. Verified. 64.1k+ views. Hint: Generally the molecular orbital diagrams are used to understand the bonding of a diatomic molecule. You should know that molecular orbital diagrams are used to deduce magnetic properties of a molecule; they also ...
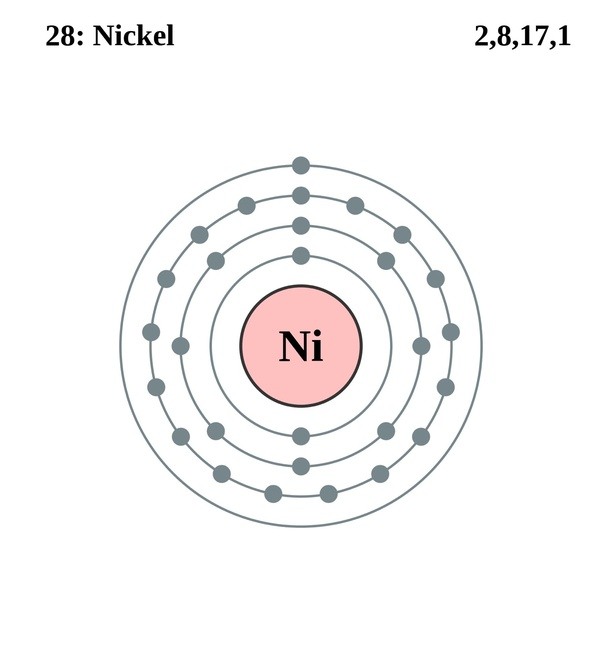
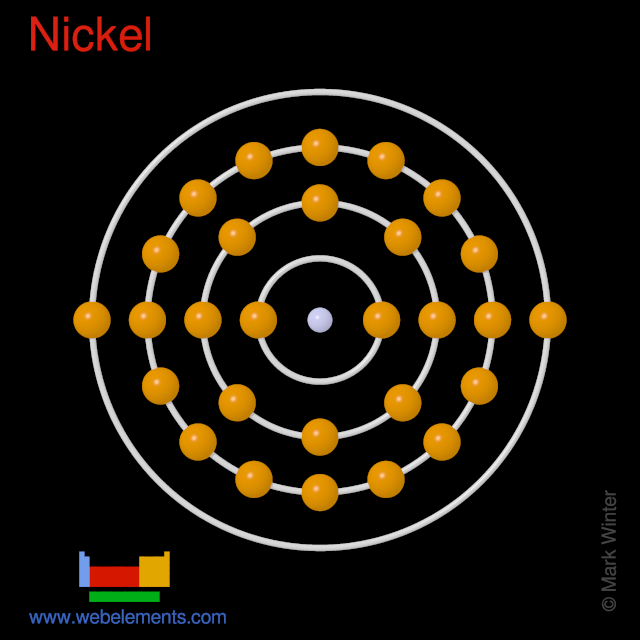
Oxidation States, +2,3. Electrons Per Shell, 2 8 16 2. Electron Configuration, [Ar] 3d8 4s2. 1s2 2s2 2p6 3s2 3p6 3d8 4s2. Orbital Diagram.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Walsh diagram OH 2, SH 2, NH 2 8 Bent-, FH2 + NH 2, PH 2, CH 2 7 Bent-, OH2 + CH 2, SiH 2, BH 2 6 Bent-, NH2 + BH 2, AlH 2, CH 2 5 Bent BeH 2, BH 2+ 4 Linear LiH 2, BeH 2+ 3 Linear LiH 2+ 2 Bent No. of Shape valence electrons Molecular species Known shape of some AH 2 molecules Recall: a molecule adopts the structure that best stabilises the HOMO.
Their blank d -splitting diagrams within the realm of crystal field theory are: [Ni(CN)4]2−: The d orbitals fill with 8 electrons, then, with a low spin configuration. You can see that an even number of d orbitals will get filled ( dyz,dxz,dz2,dxy) with an even number of 3d electrons. This gives rise to a diamagnetic configuration, as expected.
• MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
Problem: Construct the orbital diagram for Ni. FREE Expert Solution. Ni → atomic # 28 → 28 electrons. Ni will pass through 1s, 2s, 2p, 3s, 3p, 4s2, 3d. Following Aufbau principle (fill lowest energy first) and Hund's rule (half-filled first before totally filled) 82% (205 ratings)
Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals 1. Begin with the Lewis structure. 2. Decide how many orbitals each atom needs to make its sigma bonds and to hold its non-bonding electrons. Draw the atomic and hybrid orbitals on on side of the page. 3. For each sigma bond, take a hybrid (or atomic) orbital from ...
Question: Construct the orbital diagram for Ni. Construct the orbital diagram for Ni. see more. Best answer. Atomic orbital diagrams are also known as electron-in-a-box diagrams. These are simplified diagrams of how electrons are arranged within the orbitals for a. Answer to Construct the orbital diagram for Ni. Start by adding the appropriate ...
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Jul 14, 2016 — Nickel is 3d8 4s2. The 4s orbital is fully occupied even though it is higher in energy. This is due to inter electron repulsion within 3d which effectively " ...4 answers · 5 votes: Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules ...What is the orbital diagram for chromium?1 answerAug 20, 2015What is the orbital diagram for magnesium?1 answerAug 20, 2015More results from www.quora.com
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Construct the orbital diagram for nickel. Answer Bank 111 Energy.
Jan 26, 2021 — Valence electrons are the electrons which are located in the outer shell or orbit. There are 28 electrons in the nickel in the 4 orbits and the ...
DOWNLOAD Wiring Diagrams Free. Close DOWNLOAD. Construct The Orbital Diagram For Ni. More Details . 76 Plymouth Duster Dome Light Wiring Diagram. More Details . 2003 Envoy 4.2 Liter Cooling Fan Wiring Diagram. More Details . Jvc Kd-g230 Wiring Diagram. More Details . Warwick Corvette Wiring Diagram.
Construct the orbital diagram for ni. Construct the orbital diagram for ni. These are simplified diagrams of how electrons are arranged within the orbitals for a. The metal is produced by heating the ore in a blast furnace which replaces the sulfur with oxygen. Draw the orbital diagram for ion co 2.
(ii) Complete the diagram to show the splitting of the d orbital energy levels in an octahedral complex ion. energy (iii) On the axes below, sketch the shapes of one d orbital from the lower energy level and one d orbital from the higher energy level. y lower energy level z x y z x higher energy level [4]
2. Molecular orbital theory: This is the best model to explain the bonding within the CO ligand as well as in metal carbonyl complexes. There are total three molecular diagrams for carbonyl ligand which were proposed from time to time. Though, all three molecular orbital (MO) diagrams are able to explain the nature of metal-4




















0 Response to "38 construct the orbital diagram for ni"
Post a Comment