37 orbital diagram of phosphorus
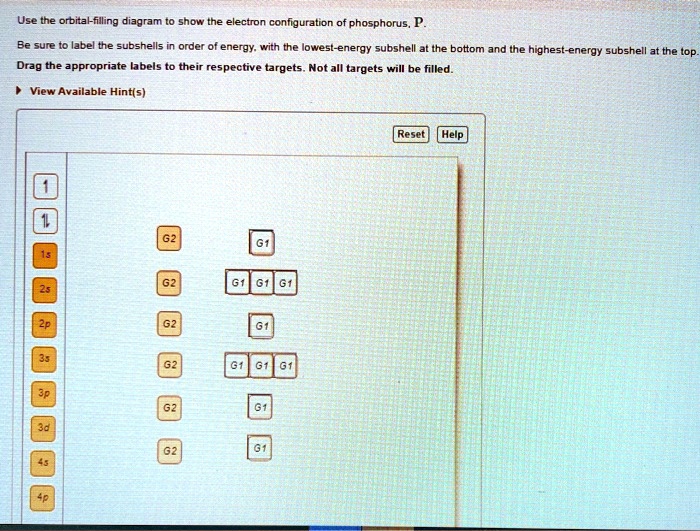
You are watching: Use the orbital-filling diagram to show the electron configuration of phosphorus, p. Electron Configuration. Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons. PLEASE HELP! Write the full electron configuration for phosphorus, atomic symbol P, then draw an orbital box diagram that accounts for all of the electrons in phosphorus. You don't need to include the orbital box diagram as part of your answer. Based on your drawing, explain why phosphorus is either paramagnetic or diamagnetic.
Use the orbital-filling diagram to show the electron configuration of helium, He. ... phosphorus silver molybdenum. phosphorus germanium silver molybdenum strontium. Arrange the following elements in order of decreasing metallic character (high to low): Cl Cs Sr Rh Se Mo As Cd. Cs Sr Mo Rh Cd As Se Cl.
Orbital diagram of phosphorus
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. A molecular orbital diagram or MO diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method (LCAO method) in particular.schematron.org: Phosphorus: Orbital and Bonding InfoWhat is the orbital ... Orbital diagrams are pictorial descriptions of the electrons in an atom. Three guidelines are helpful in forming orbital diagrams. In response to the Auf Bau Precept, every electron occupies the bottom vitality orbital. You leap up a bit bit in vitality and we get the 2s orbital that make it the 2p sublevel.
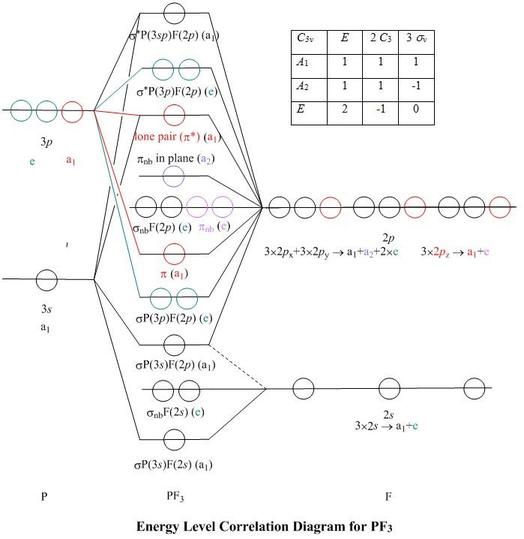
Orbital diagram of phosphorus. The whole point of that was to see how the oxygen orbital energies split up (and which were two-fold or three-fold degenerate). It was also to figure out which orbitals on phosphorus interact with which orbitals on the oxygen atoms. The resultant MO diagram was: Takeaways: This is only qualitative, so take it with a grain of salt. Orbital Diagram Of Phosphorus. what is the orbital diagram for phosphorus the orbital diagram for phosphorus consists of five electrons in the third shell eight in the second and two in the first shell closest to the nucleus electron configuration for phosphorus p the next six electrons will go in the 2p orbital the p orbital can hold up to six electrons we ll put six in the 2p orbital and ... In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the ... Clearly, it is a phosphorus sp mixture orbital, with a visible p-type inner lobe, and contains, according to the present calculation, 37% P(3s), 32% P(3p z) and 9% from each fluorine 2p z The highest energy valence-shell MO, labelled as σ * P(3 sp )F(2 p ), has more phosphorus p -character, and contains 19% P(3 s ) and 43% P(3 p z ).
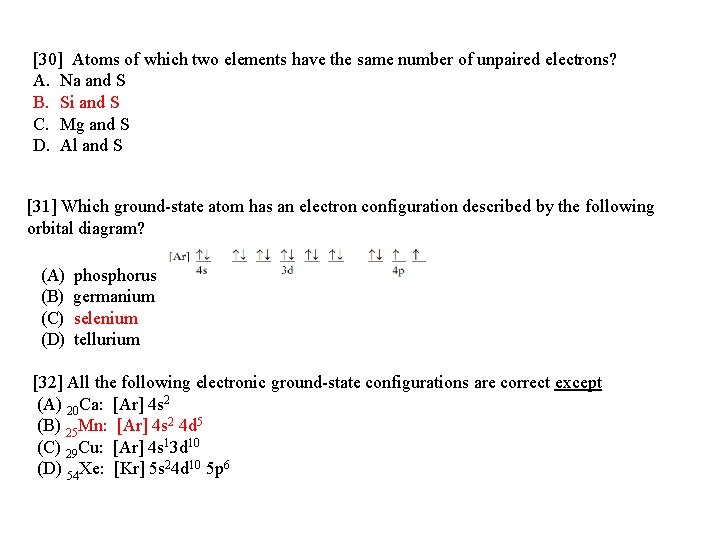
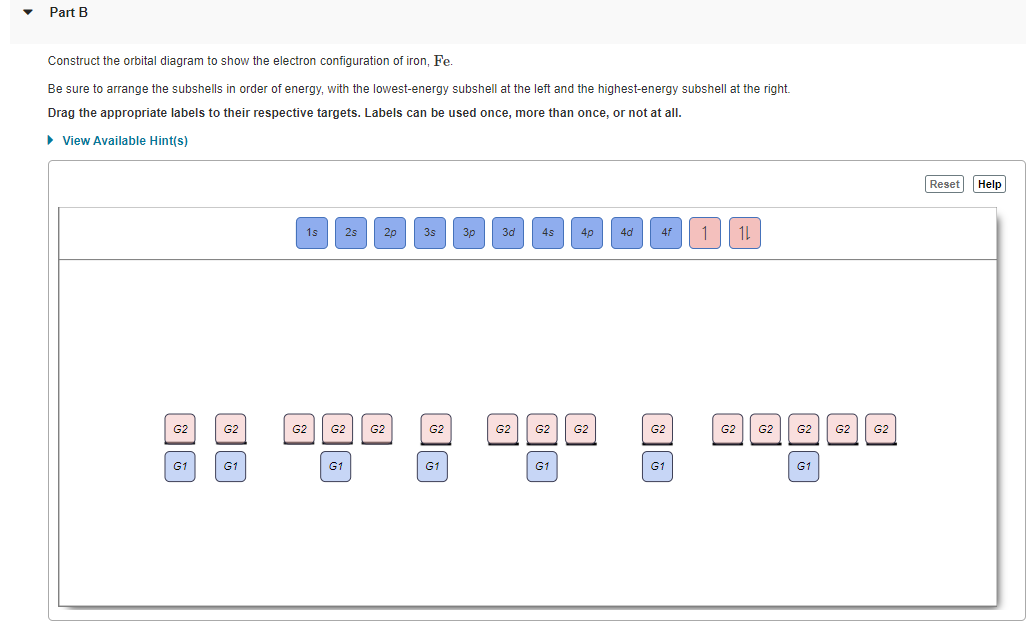
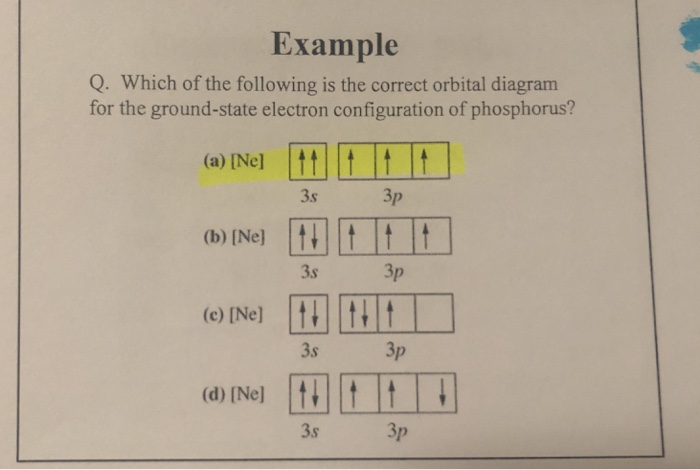
Chemistry questions and answers. Question 17.a of 25 Submit Examine the orbital diagram for the ground state electron configuration of phosphorus. Choose the correct orbital diagram for the ground state electron configuration of phosphorus. 1) [Nej 11 3s Зр A) II) [Ne) 11 B) II 3s 3p [Ne] C) III 11 3s 3p D) IV IV) [Ne] 11 + 3s 3p. Draw an orbital diagram and use it to derive the electron configuration of phosphorus, Z = 15. What is its valence electron configuration? Given: atomic number. Asked for: orbital diagram and valence electron configuration for phosphorus. Strategy: Locate the nearest noble gas preceding phosphorus in the periodic table. Draw an orbital diagram and use it to derive the electron configuration of phosphorus, Z = 15. What is its valence electron configuration? Given: atomic number. Asked for: orbital diagram and valence electron configuration for phosphorus. Strategy: Locate the nearest noble gas preceding phosphorus in the periodic table. What is the atomic structure of phosphorus? Diagram of the nuclear composition and electron configuration of an atom of phosphorus-31 (atomic number: 15), the most common isotope of this element. The nucleus consists of 15 protons (red) and 16 neutrons (blue). 15 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
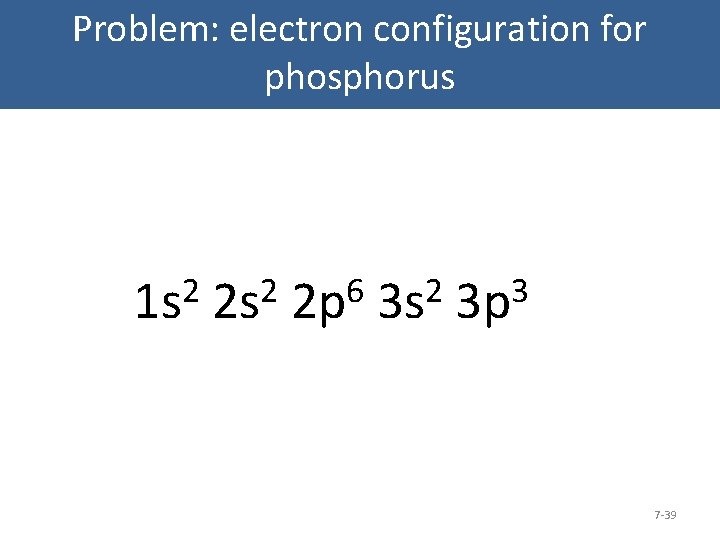
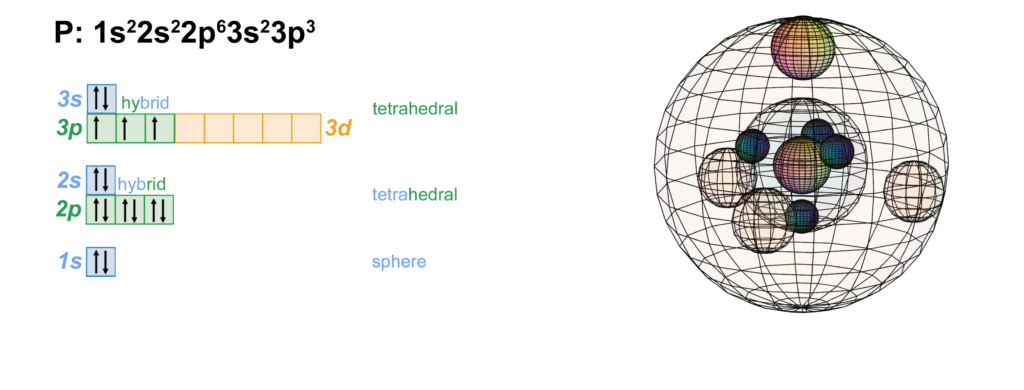
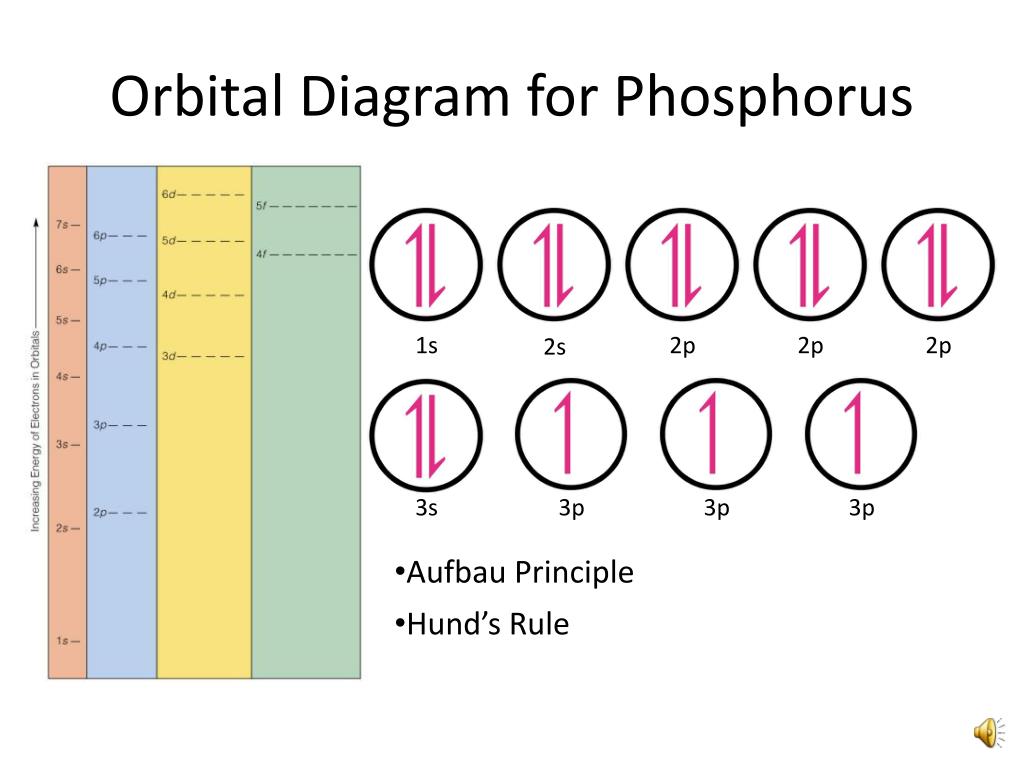
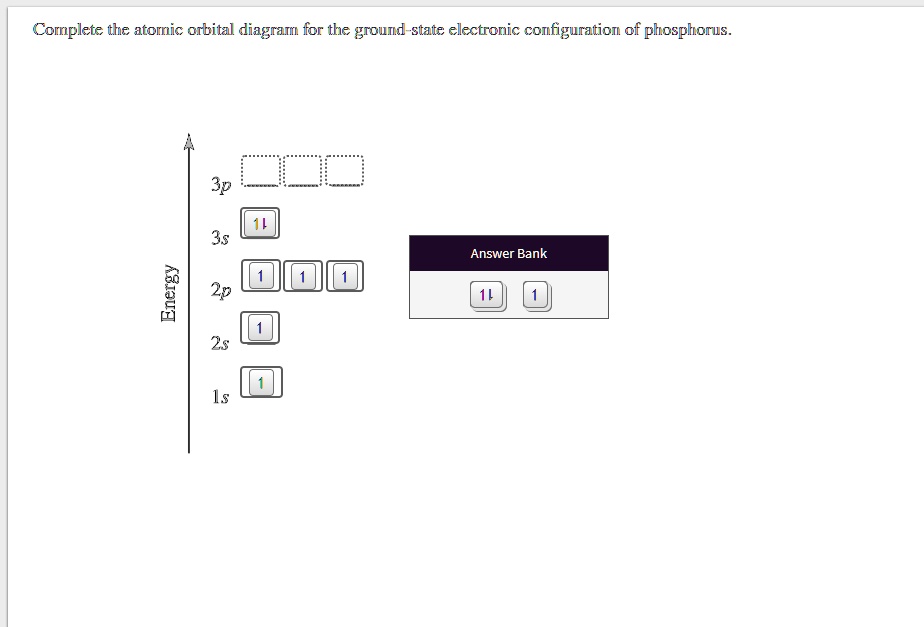
There are alkali, argon, electron, nitrogen, phosphorus, silicon, and sulfur orbital diagrams that you can save for free. Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. P (Phosphorus) is an element with position number 15 in the periodic table. Located in the III period. Melting point: 44 ℃. Density: 1.82 g/cm 3 . Electronic configuration of the Phosphorus atom: 1s 2 2s 2 2p 6 3s 2 3p 3. Reduced electronic configuration P: [Ne] 3s 2 3p 3. Below is the electronic diagram of the Phosphorus atom Distribution of ... Irrespective of it, the phosphorus trifluoride (PF3) shows pi bonding characteristics due to sp3 hybridization and back-bonding. The detail of how hybridization is taking place can be studied through the molecular orbital diagram of phosphorus trifluoride (PF3) molecule. Complete the atomic orbital diagram for the ground-state electronic configuration of phosphorus. 3p C00 Answer Bank Answer Bank Energy 2,0 ; Question: Complete the atomic orbital diagram for the ground-state electronic configuration of phosphorus. 3p C00 Answer Bank Answer Bank Energy 2,0
The aufbau diagram shows the. The atomic number of phosphorus is Write the electron configuration of a phosphorus atom. 1s22s22p63s23p3. You can obtain correct electron configurations for the elements up to. In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital.
Answer (1 of 3): You want a Walsh diagram for \mathrm{AH_3}. The molecular orbitals for \mathrm{PH_3} are on the right-hand side. The highest occupied orbital (HOMO) is \mathrm{2a_1}, but this diagram does not show it as occupied because it is using electrons for e.g. \mathrm{AlH_3}. (The reason ...
Orbital Diagrams: Orbital diagrams show the distribution of electrons in the electron shells and subshells of an atom. A few principles and rules must be followed in this process.
Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus (P) 16: Orbital diagram of Sulfur ...
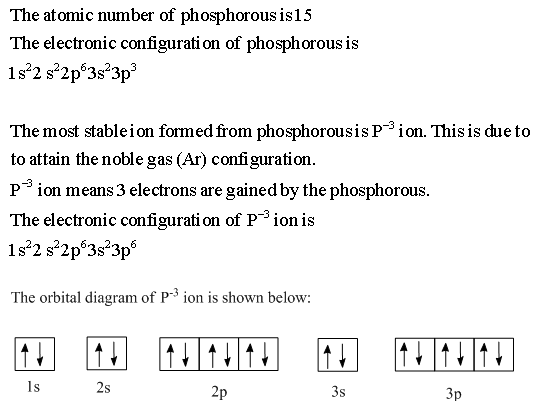
Build the orbital diagram for the ion most likely formed by phosphorus? 1s22s22p63s23p3 is for Phosphorus and the most likely ion is to be a 3- because it wants to have a full outer shell ...
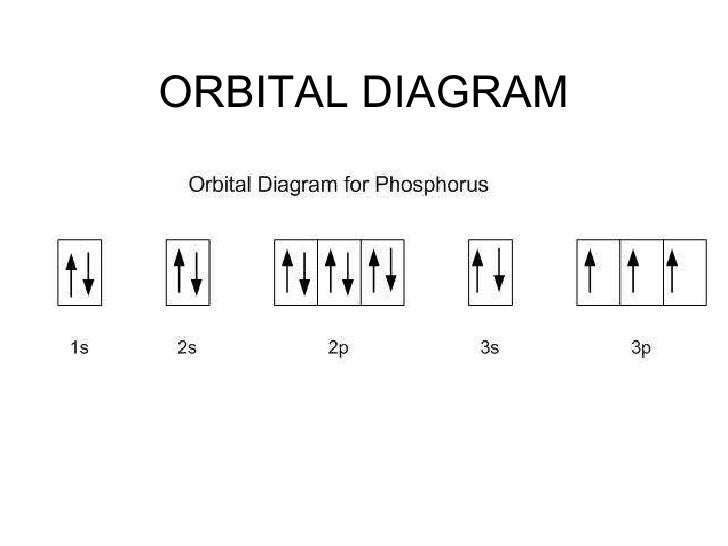
Draw orbital diagrams for the following elements: 1. phosphorus. ↑↓. ↑↓. ↑↓ ↑↓ ↑↓. ↑↓. ↑ ↑ ↑. 1s. First, he Aufbau principle requires that lower energy orbitals are filled with Since phosphorus in a third period element, the first (K) and second (L) shells are . Phosphorus, P, is located in period 3, group 15 of ...
The remaining one is a non-bonding orbital but doubly field, which denotes the lone pair of phosphorus. Given below is the MO diagram of PF3 taking reference to which you can easily draw for PCl3. A MO diagram helps us to know about the bonding, bond order, bond angle, and bond length of any compound.
The atomic number of phosphorus is 15. It has 5 valence electrons in its valence shell. The orbitals involved in phosphorus atoms are 1s, 2s, 2p, 3s, 3p. The outermost orbitals, 3s and 3p have 5 ...
HYPERVALENCY OF PHOSPHORUS. Since phosphorus (#"P"#, atomic number #15#) is on the third period of the periodic table, it has access to orbitals of principal quantum number #n = \mathbf(3)#.That means it can use its #3d# orbitals in addition to its typical #3s# and #3p# valence orbitals.. This generates an octahedral molecular and electron geometry.You can see the final shape of this at the ...
Each orbital can hold 2 electrons and each arrow. The order of filling of the energy levels is 1s 2s 2p 3s 3p 4s. The image below is the orbital diagram for phosphorous. Valent and pentavalent phosphorus. Phosphorus atomic orbital and chemical bonding information. Molecular Geometry of PCl5. The p orbital can hold up to six electrons.
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three guidelines are helpful in forming orbital diagrams. In response to the Auf Bau Precept, every electron occupies the bottom vitality orbital. You leap up a bit bit in vitality and we get the 2s orbital that make it the 2p sublevel.
A molecular orbital diagram or MO diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method (LCAO method) in particular.schematron.org: Phosphorus: Orbital and Bonding InfoWhat is the orbital ...
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.






















0 Response to "37 orbital diagram of phosphorus"
Post a Comment