37 lewis dot diagram for iron
Lewis Structure of Iron (II) Oxide, FeO. Iron (II) oxide's Lewis Structure is among one of the easiest to draw. The iron atom, because it has a +2 charge in this compound, is drawn with two valence electrons - and since it is a metal, it wants to give them away ("lose them"). Oxygen, by contrast, is a non-metal with six valence ...
The Lewis dot diagram for Iron is the letters FE with seven dots around it, with no more than 2 dots on each side. Iron is not a part of the halogen family. Good try! However, the electron...
SeO2 Lewis Structure, Geometry, Hybridization, and Polarity. The chemical formula of selenium dioxide is SeO2. It is a unidimensional polymer chain having alternating selenium and oxygen atoms. This chemical compound is of great importance because of its corrosive nature for metals only when in contact with water.
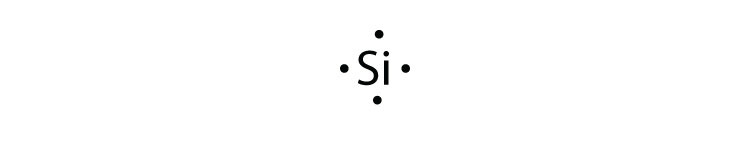
Lewis dot diagram for iron
The Lewis dot structure for a sulfate ion gives sulfur a formal charge of zero with six bonds. Based on the structure and the Lewis dot theory, which of the following statements about the sulfur atom is false? ... Steel—a solid solution consisting of carbon atoms seated in the holes of an iron atom structure—is an example of a(n) Choose one ...
Lewis Dot Diagrams Dot Diagrams (sometimes known as Lewis dot diagrams) are a depiction of an atom's valence electrons. They are a powerful tool in helping you understand, see, and even predict molecular bonding. The dots represent valence electrons Neon has 8 valence electrons and no openings. Neon has fulfilled the octet rule and will
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ...
Lewis dot diagram for iron.
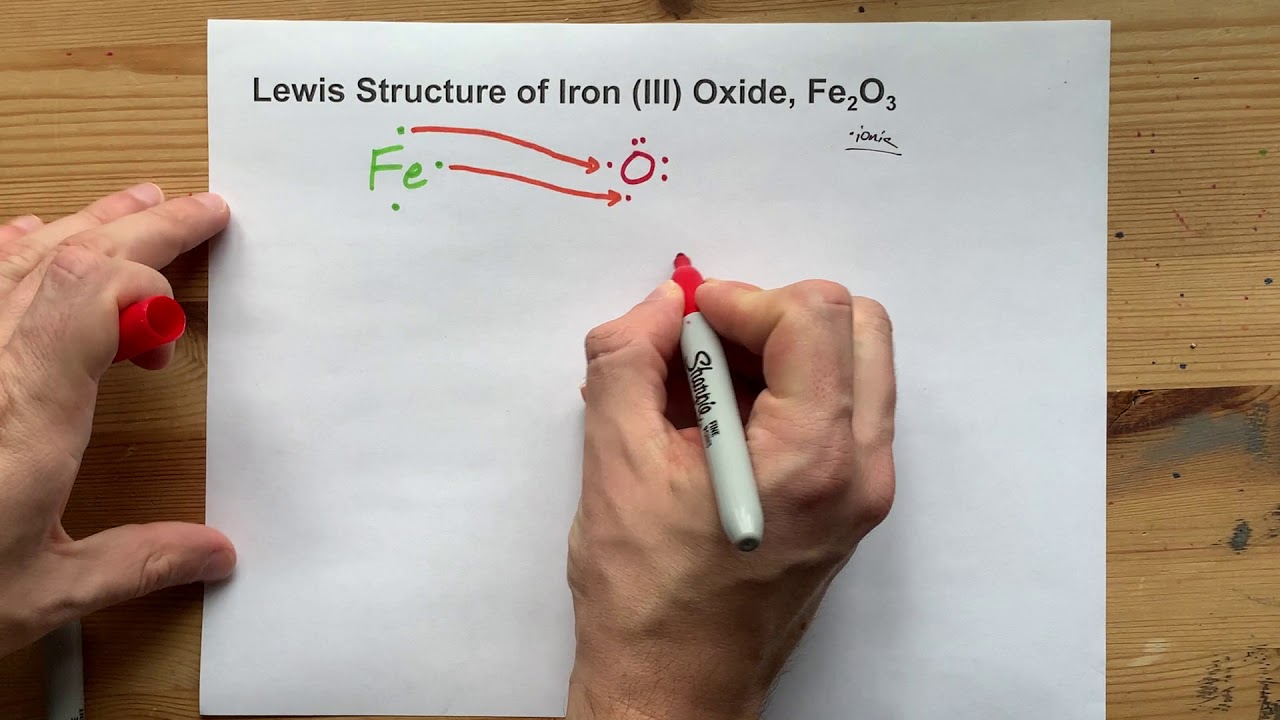
Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The color depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red.
Draw the Lewis Dot Structure. Notes: Scientists use. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons.
sodium to chlorine can be depicted by a Lewis dot diagram. Na.Cl. Calcium would need two chlorine atoms to get rid of Its two valence electrons. Show the transfer of electrons In the following comblnatlons. 3. Be + S 4. Na + O Al+3 Page 38
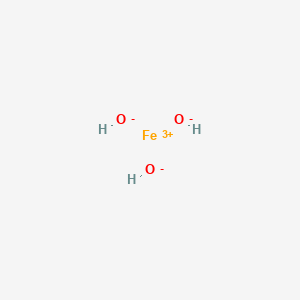
A step-by-step explanation of how to draw the Fe(OH)2 Lewis Dot Structure.For Fe(OH)2 we have an ionic compound and we need to take that into account when we...
I know how to draw a Lewis Dot Diagram. Atomic Structure Worksheet. Fill in the blanks for the elements in this chart. Element Symbol. Element. Number of Protons. Number of Neutrons. Number of Electrons. ... Iron. Lead. Tin. Oxygen. Helium. Chlorine. For the following elements, show the abbreviated (Noble Gas) electron configurations. Iodine ...
Draw Lewis dot diagrams of the following atoms. 2. Carbon 3. Neon 1. Calcium 4. Hydrogen Ionic bonding occurs when a metal transfers one or more electrons to a nonmetal in an effort to attain a stable octet of electrons. For example, a Lewis dot diagram can depict the transfer of an electron from otassium to chlorine.
Lewis Structures for Polyatomic Ions. When writing dot structures for polyatomic ions, you must remember to add or subtract the amount of electrons represented by the charge. When writing polyatomic ions, you must include the structure inside brackets, [ ], with the charge outside the bracket
Answer (1 of 2): Lewis dot formula had been invented to represent the number of electrons in the outermost shell (Valence shell) for the main group elements, to figure out the number of electrons which are needed to be lost or shared or gained by the element to complete its octet and to be used i...
Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral.
Draw two Lewis (electron dot) structures for BrO3- [1] Structure II —> does not follow octet rule ... Lewis structure with formal charges closet to 0 provides the greatest stability for the structure. Structure II has FC of 0 and structure I has FC of 2+ ... Draw the abbreviated orbital diagram for an iron atom using the arrow-in-box notation ...
answer choices. Electrons are attracted to the nucleus of the central atom. Shared and unshared electron pairs repel each other as much as possible. Molecules repel one another due to intermolecular forces. There is always an octet of electrons around an atom in a molecule.
Electron Dot Diagrams There is another model called the electron dot or Lewis diagram. This system represents an atom and its valence electrons. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. The electrons in the valence shell are shown as dots placed around the symbol.
Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. A chemical formula is a way of expressing information about the proportions of atoms that constitute a ...
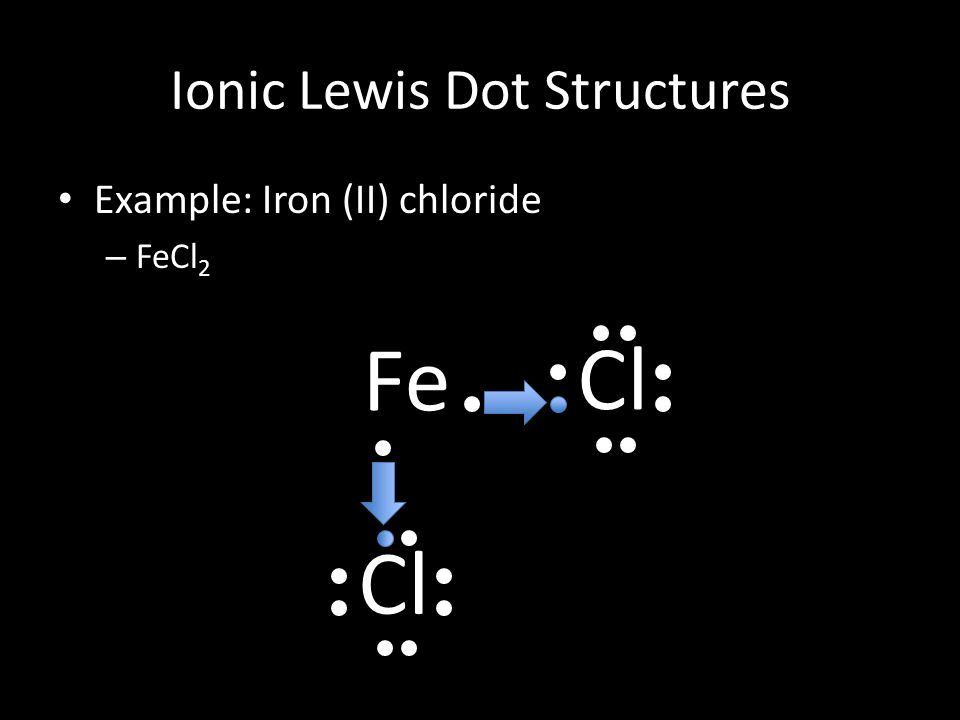
The given compound ferric bromide, FeBr3 F e B r 3 , is an ionic compound. In this substance, iron has a charge of 3+ in ferric cation, Fe3+ F e 3 + , which means that it has lost 3 electrons. We ...
If you are asked to write the Lewis Structure of Iron (Fe) you'll first need to find the number of valance electrons for Iron. One of the challenges in writi...
Draw Lewis dot diagrams with arrows to show how iron(III) chloride is formed by ionic bonds. Remember, arrows show the transfer of valence electrons. 12. Cyanide chemical suffocation interferes with the body using what gas: hydrogen /oxygen / nitrogen? 13. gas. (Covalent bonding)Draw the Lewis structure for H 2 14. Draw the Lewis structure for ...
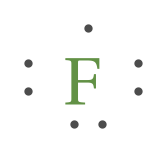
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Answer (1 of 2): Instead of acting like an entitled egomaniac who thinks he's the smartest guy in the room like the other guy did, I'll provide an actual answer. Since nickel is a transition element, you have to manually write out its electron configuration and figure out how many electrons the l...
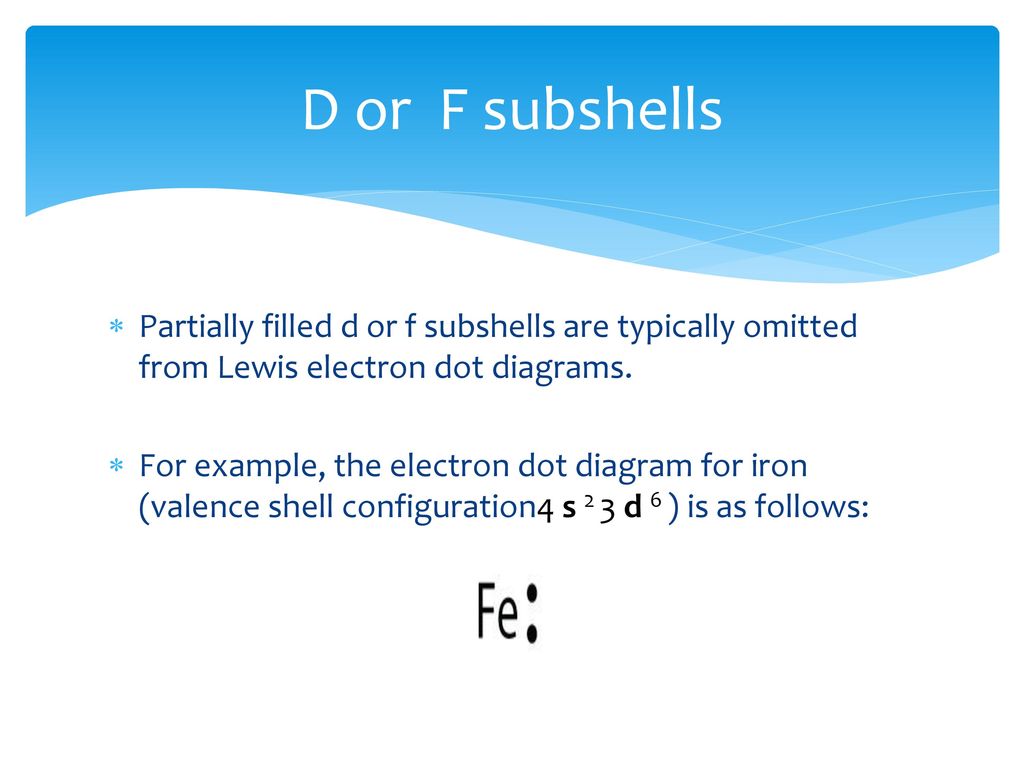
What is the Lewis electron dot diagram for each element? 1. phosphorus 2. argon D or F subshells ∗ Partially filled _____ subshells are typically omitted from Lewis electron dot diagrams. ∗ For example, the electron dot diagram for iron (valence shell configuration _____) is as follows: Periodic Table Columns
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration.
What is the Lewis electron dot diagram for each ion? Ca 2+ O 2- Solution Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+ . Ca 2+ The O 2- ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows:
Lewis dot structure for iron. Judging from the valence shell structure (in which I could include also the 3d- orbital electrons, because the energy difference between 3d and 4s is so small), I ...
*Iron does not follow the rules in determining the Lewis diagram. This is a special case and unless you are an expert, you would not be expected to figure this out by yourself. Write the Lewis symbols for each atom. See graphic on the left.




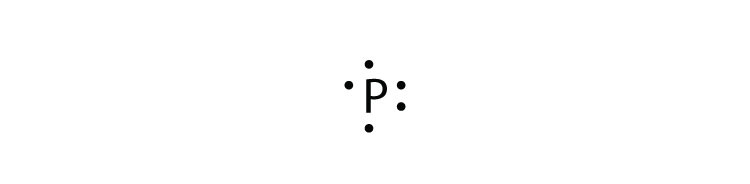
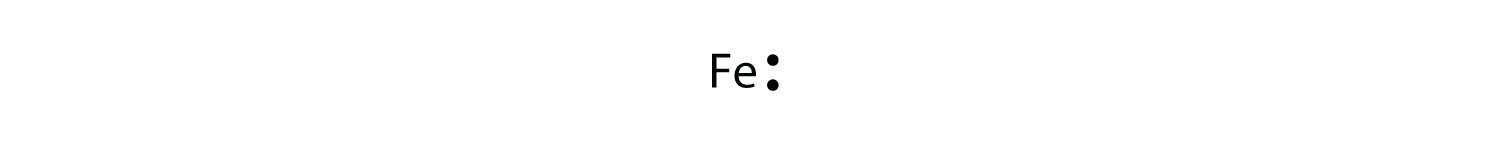
:max_bytes(150000):strip_icc()/Iron-58b602243df78cdcd83d3d5a.jpg)






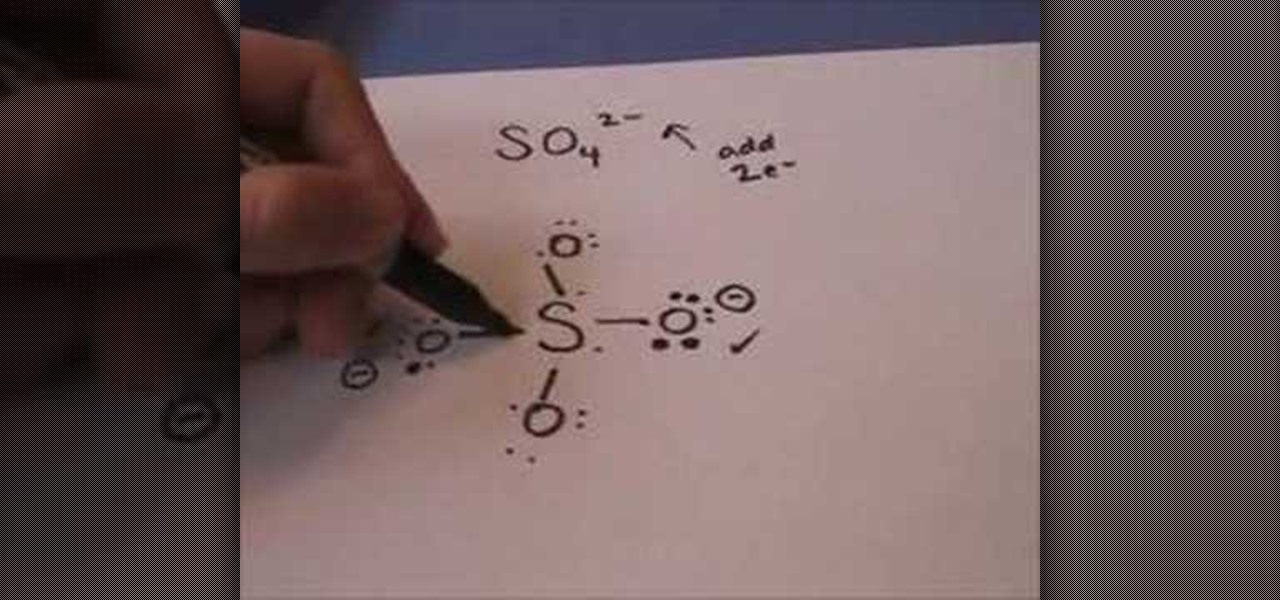






0 Response to "37 lewis dot diagram for iron"
Post a Comment