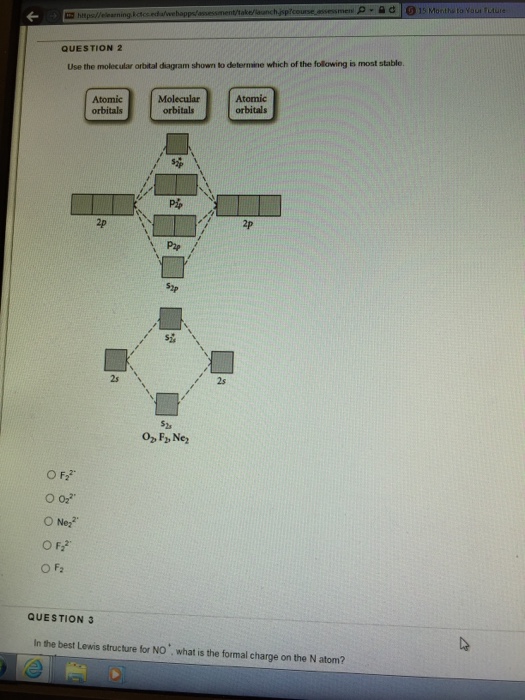
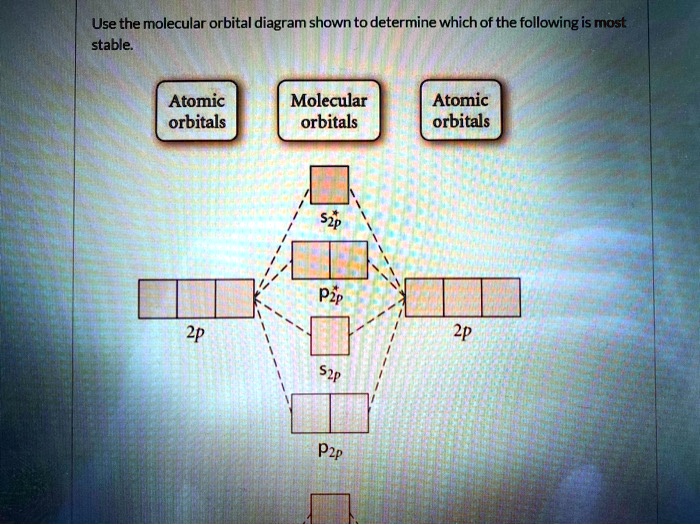
35 use the molecular orbital diagram shown to determine which of the following is most stable.
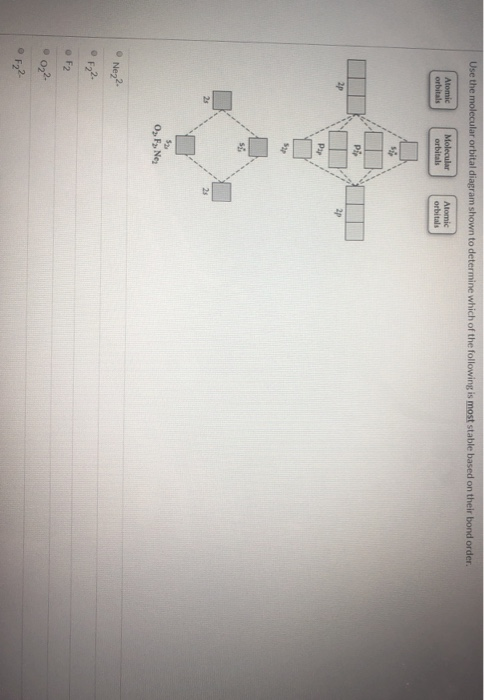
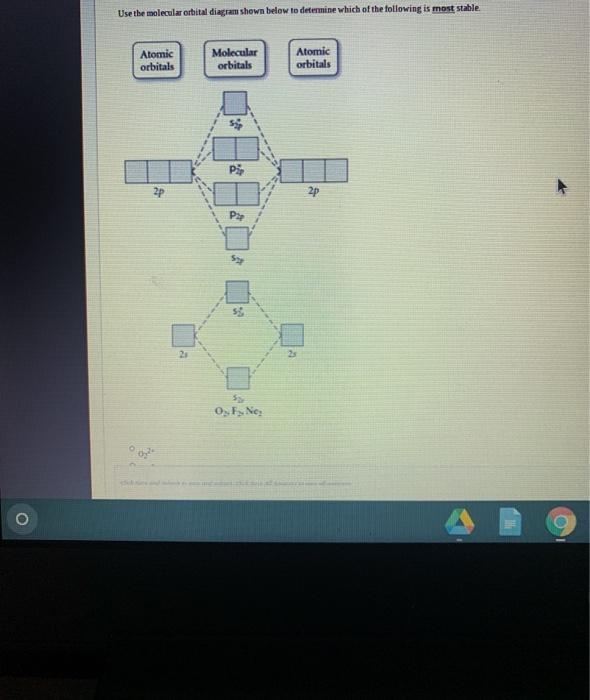
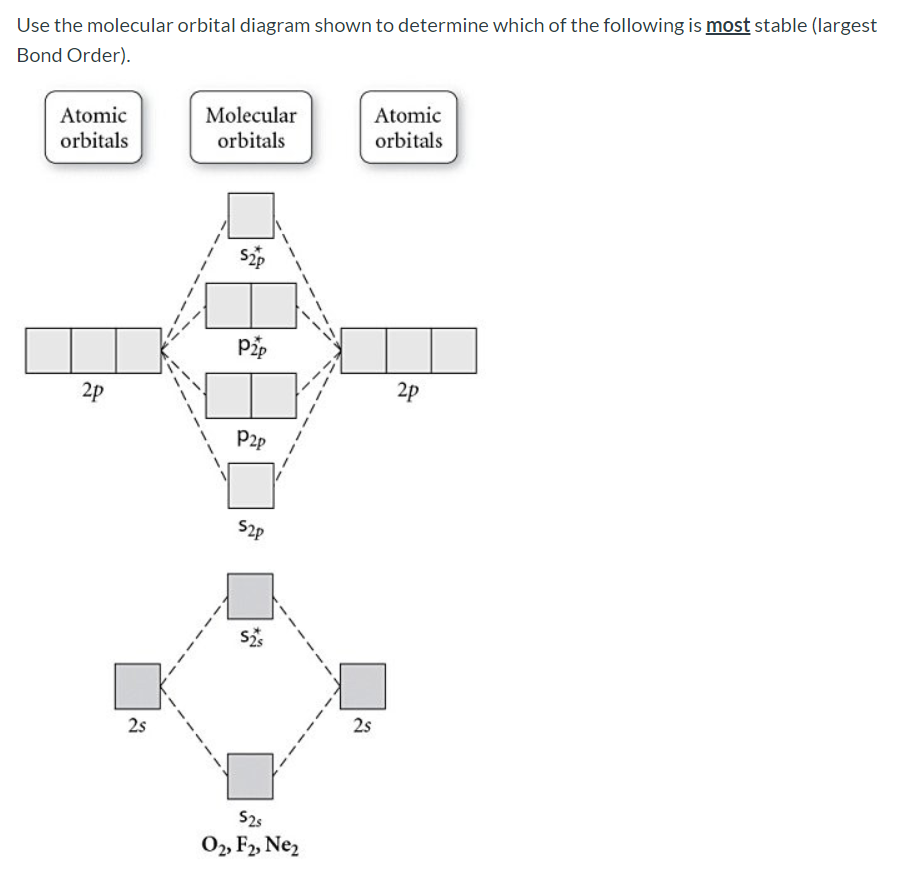
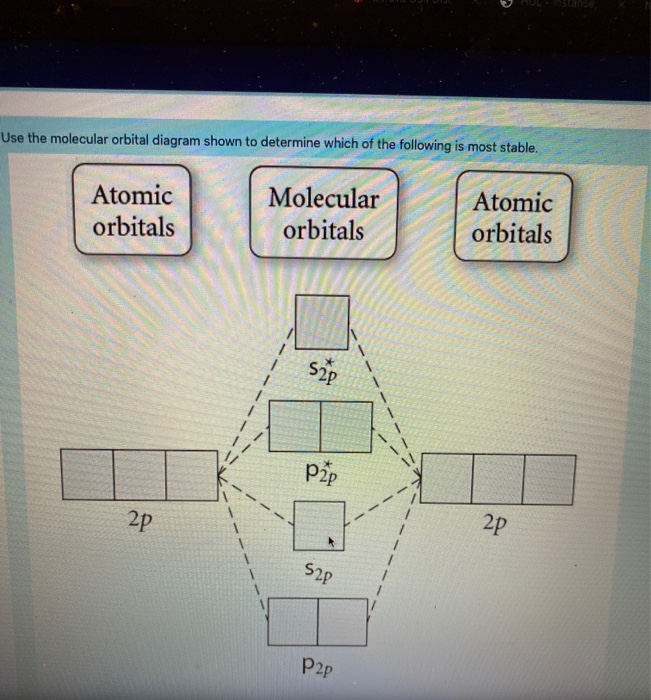
Use the molecular orbital diagram shown to determine which of the following is most stable based on their bond order. Atomic orbitals Molecular orbitals Atomic orbitals O, F, Ne Ne22 F₂2. F2 . 022- • F22. Question: Use the molecular orbital diagram shown to determine which of the following is most stable based on their bond order.
Use molecular orbital diagram shown to determine which is most stable a) O22-b)F2 c) F22+ d) F22-e) Ne22-a. Use the molecular orbital diagram shown to determine which of the following is most stable. A) F2 B) F22⁺ ...
Use the molecular orbital diagram shown to determine which of the following is most stable. A. F22+ B. Ne22+ C. F22- D. O22+ E. F2.
Use the molecular orbital diagram shown to determine which of the following is most stable.
Problem Details. Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A. Ne 22+ B. O 22+ C. F 22+ D. O 22- E. None of the above are paramagnetic. Learn this topic by watching MO Theory: Homonuclear Diatomic Molecules Concept Videos.
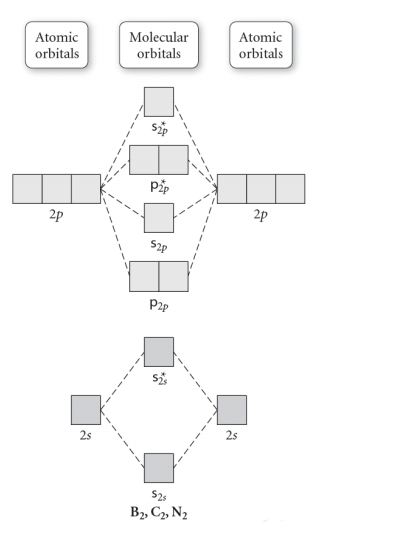
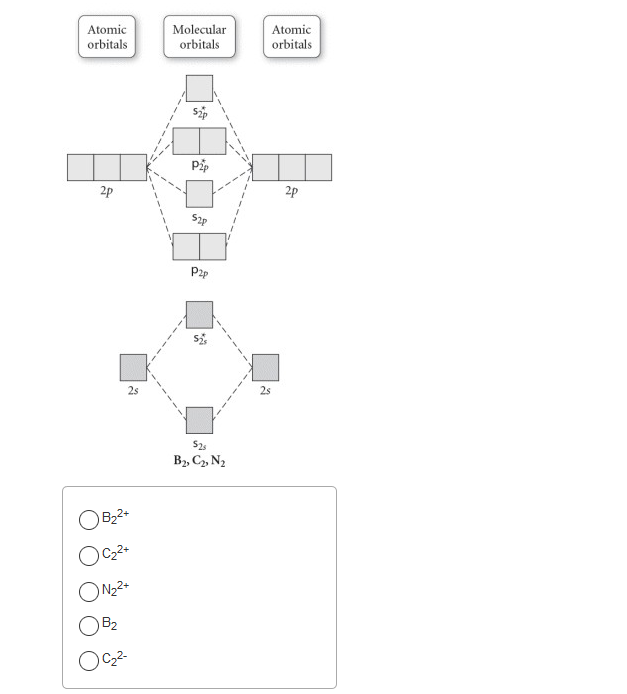
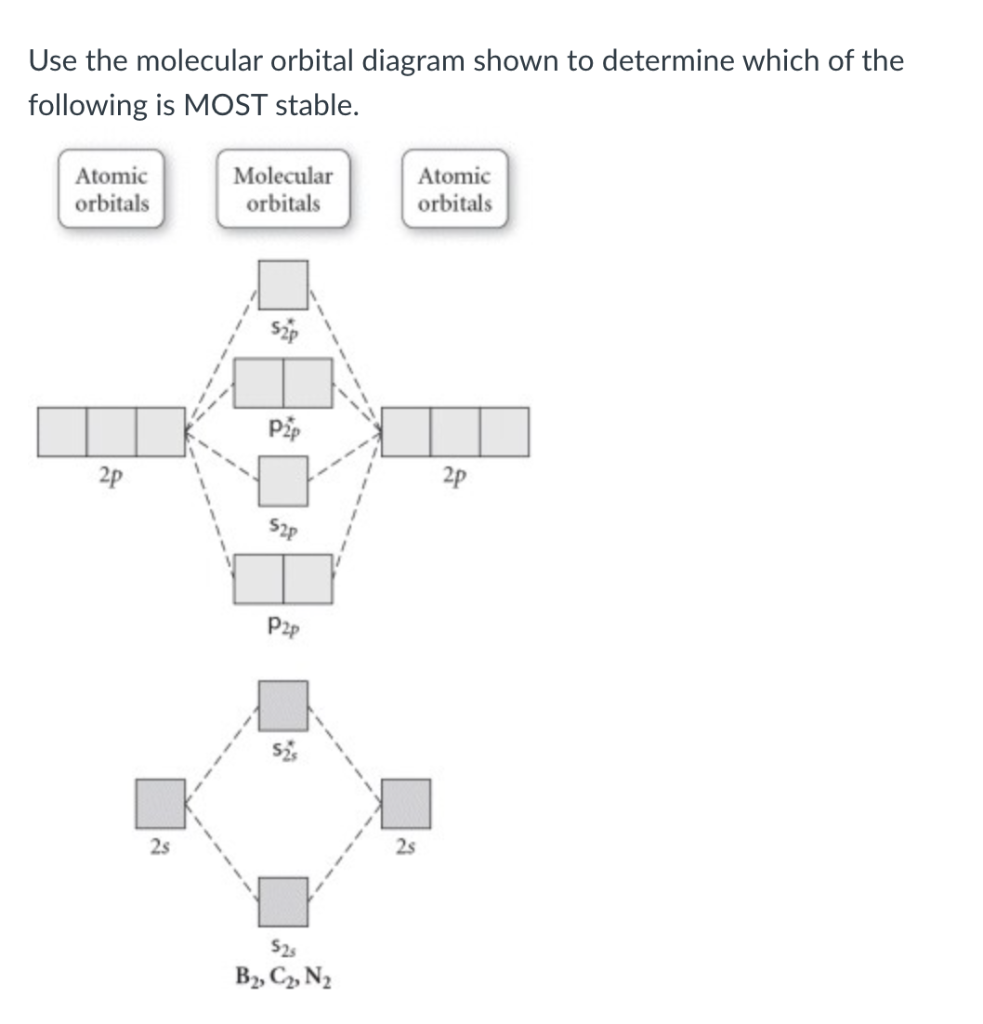
Use the molecular orbital diagram shown to determine | Chegg.com. Science. Chemistry. Chemistry questions and answers. Use the molecular orbital diagram shown to determine which of the following is most stable. Atomic orbitals Molecular orbitals Atomic orbitals 7 Sp 2p 2p 2p TT 3 2s 25 B, C, N, 2 2 N2 B2 C2 2 B2 C22.
Problem Details. Use the molecular orbital diagram shown below to determine which of the following diatomic species has the highest bond order. a. F 22⁻ b. F 22⁺ c. O 22⁺ d. F 2 e. Ne 22⁺. Learn this topic by watching MO Theory: Bond Order Concept Videos. All Chemistry Practice Problems MO Theory: Bond Order Practice Problems.
Use the molecular orbital diagram shown to determine which of the following is most stable..
Chemistry questions and answers. Part A Use the molecular orbital diagram shown to determine which of the following is most stable Atomic orbitals Molecular orbitals Atomic orbitals TE 2p 2p 22 Tap 25 BCN NA? 82 MacBoo Use the molecular orbital diagram shown to determine which of the following are paramagnetic Atomic orbitals Molecular orbitals ...
Use molecular orbital diagram shown to determine which is most stable a o22 bf2 c f22 d f22 e ne22 a. A asdfasdf b asdfasdf c asdf d f2 2 e none of the above are paramagnetic. Label each and each and every molecular orbital with its call sigma pi and position the accessible electrons interior the perfect atomic orbitals and molecular orbitals.
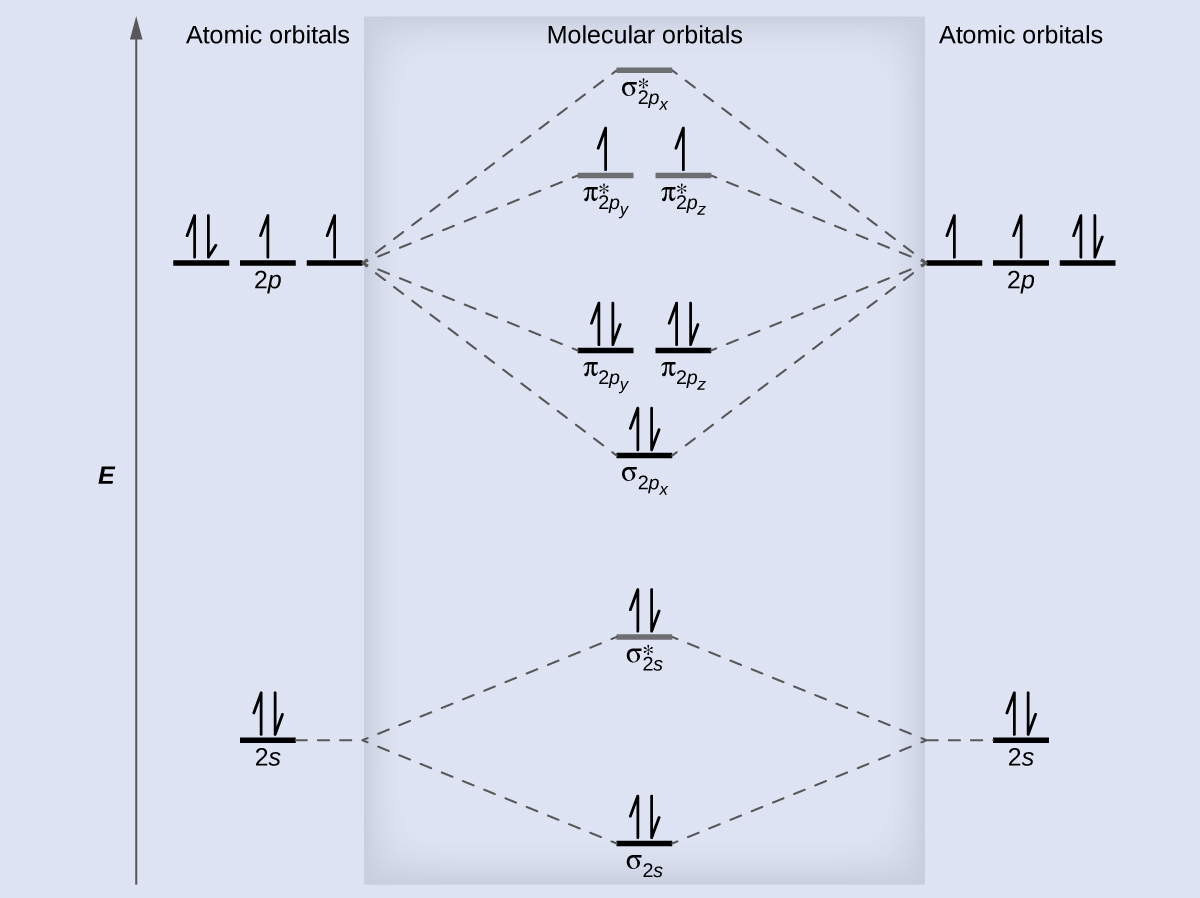
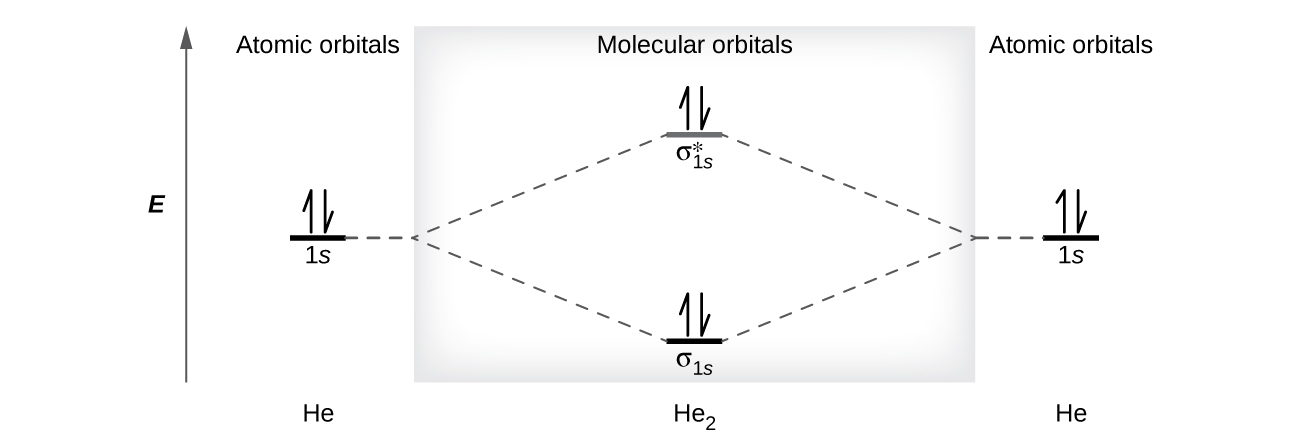
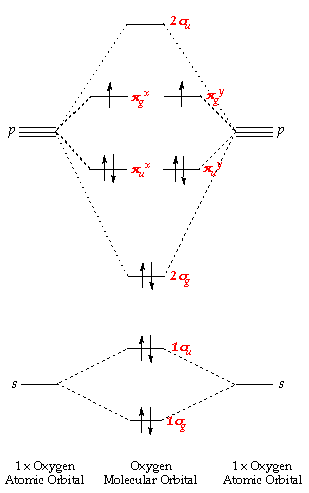
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
28 Apr 2017 — Use the molecular orbital diagram shown to determine which of the following is most stable. A. F22+ B. Ne2… Get the answers you need, now!2 answers · Top answer: Answer:The most stable element based on the molecular orbital diagram is [tex]\text ...
Use the molecular orbital diagram shown to determine which of the following is most stable. Use the molecular orbital diagram shown to determine which of the following is most stable. D c 2 2. Start studying exam 3. Inorganic Chemistry Why Do Compounds Like Sf6 And Sf4 Exist But What Is The Molecular Orbital Diagram For O2 And O2 Ions Quora
Use the molecular orbital diagram shown to determine which of the following is LEAST stable. B2⁺ The highest energy occupied molecular orbital in the F—F bond of the F2 molecule is ________.
Draw the molecular orbital diagram shown to determine which of the following is MOST stable. A) B2^2+. B) C2^2+. C) N2^2+. D) C2^2-
Use the molecular orbital diagram shown to determine which of the following is most stable. C) O2^2+ Use the molecular orbital diagram shown to determine which of the following are paramagnetic. B) B2. Identify the number of bonding pairs and lone pairs of electrons in water.
Draw the Molecular orbital Diagram Shown to Determine which Of the Following is Most Stable. use the molecular orbital diagram shown to determine which use the molecular orbital diagram shown to determine which of the following is most stable 38 a b2 b c22 use the molecular orbital question draw the molecular orbital diagram shown to answer to draw the molecular orbital diagram shown to ...
Chemistry questions and answers. Use the molecular orbital diagram shown to determine which of the following is most stable based on their bond order Atomic orbitals Molecular orbitals Atomic orbitals O, F2, Nez • Nez2 • F₂2. • F2 022 • F22.
Use the molecular orbital diagram shown to determine which of the following is most stable.
3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+ B) N2^2+ C) B2; D) C2^2-E) B2^2+ 5) Which statement regarding stable heteronuclear diatomic ...
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. Use the molecular orbital diagram shown to determine which of the following is most stable. Identify the number of bonding pairs and lone pairs of electrons in water. When two atomic orbitals come together to form two molecular orbitals one molecular ...
Eg: H + H two 1s orbitals mix to form sigma and sigma*. Two electrons total, both occupy the sigma orbital, two more electrons in bonding than antibonding orbitals, the compound is stable. Eg: He + He; same mixing as above. Four electrons, two in the sigma, two in the sigma*. Since there are as many bonding electrons as as antibonding, there is ...
Use the molecular orbital diagram shown to determine which of the following is most stable. The three 3 component mixture shown below was spotted to a tlc plate and developed using the solvent system listed. 1 draw the molecular orbital diagrams to determine which of the following is most stable.
The molecular orbital diagrams for molecules and ions are drawn from the order of increasing energies shown in the molecular orbital configuration. Always remember that the number of molecular orbitals formed must be equal to the number of atomic orbitals that were combined in the molecule.
Multiple Choice – Choose the answer that best completes the question. Use an ... Use the following molecular orbital diagram to answer questions #10-12.

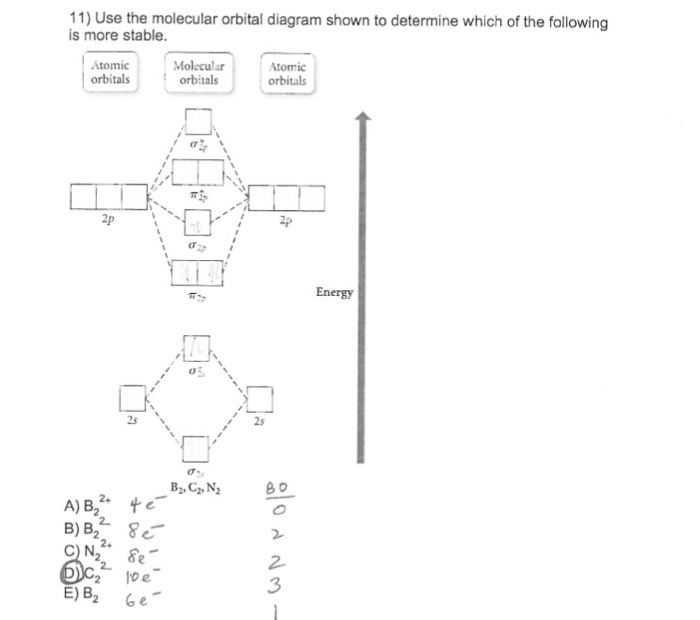
Use the molecular orbital diagram shown to determine which of the following is most stable.a) n22+ b) b2c) b22+d) c22-e) c22+
b. When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in energy than the separate atomic orbitals. c. Electrons placed in anti-bonding orbitals stabilize the ion/molecule. d.
31) Use the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2⁺ B) N2^2⁺ C) B2; D) C2^2⁻ E) B2^2⁺ 32) How many moles of oxygen are formed when 58.6 g of KNO3 decomposes according to the following reaction? The molar mass of KNO3 is 101.11 g/mol. 4 KNO3(s) → 2 K2O(s) + 2 N2(g) + 5 O2(g)
FREE Answer to Use the molecular orbital diagram shown to determine which of the following is most stable. A....1 answer · Top answer: Concepts and reason Bond order of the molecule indicates the number of bond present between the pair of atoms. Bond order is the measurement of ...
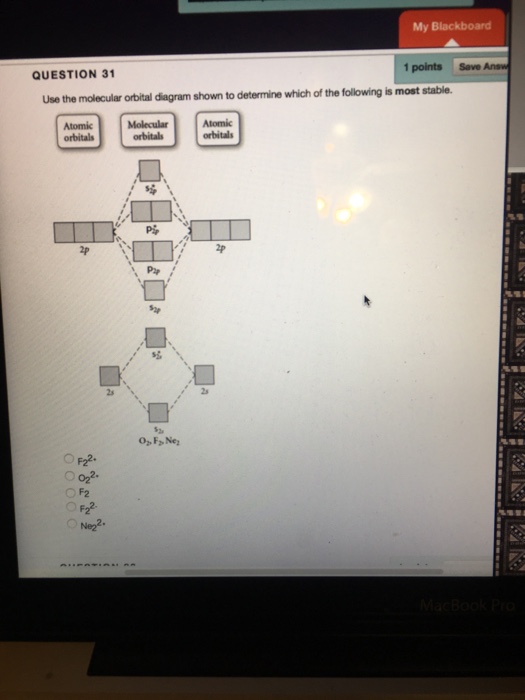
Problem: Use the molecular orbital diagram shown to determine which of the following is MOST stable. In molecular orbital names, s = sigma and p = pi.a. F2b. F22-c. O22+d. F22+e. Ne22+
Use the molecular orbital diagram shown to determine which of the following is most stable A) Ne2^2⁺ B) F2^2⁺ C) F2^2⁻ D) F2 E) O2^2⁺ E). O2^2⁺ Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A) O2 2⁺ ...
🔴 Answer: 1 🔴 on a question Use the molecular orbital diagram shown to determine which of the following is most stable. a. f22+ b. ne22+ c. f22- d. o22+ e. f2 - the answers to answer-helper.com
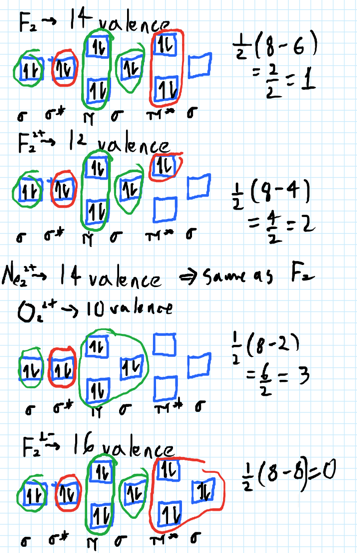
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a. F22+b. Ne22+c. F22-d. O22+e. F2. FREE Expert Solution. Recall that the bond order t ells us the strength and length of a bond: a higher bond order means the bond is stronger and shorter.
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Draw the molecular orbital diagram shown to determine which of the following is most stable. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. C22- should have the highest bond order (3, it has 6 more e ….

Use the molecular orbital diagram shown to determine which of the following are paramagnetic.a. ne22+ b. o22+ c. f22+ d. o22- e. none of the above are paramagnetic.
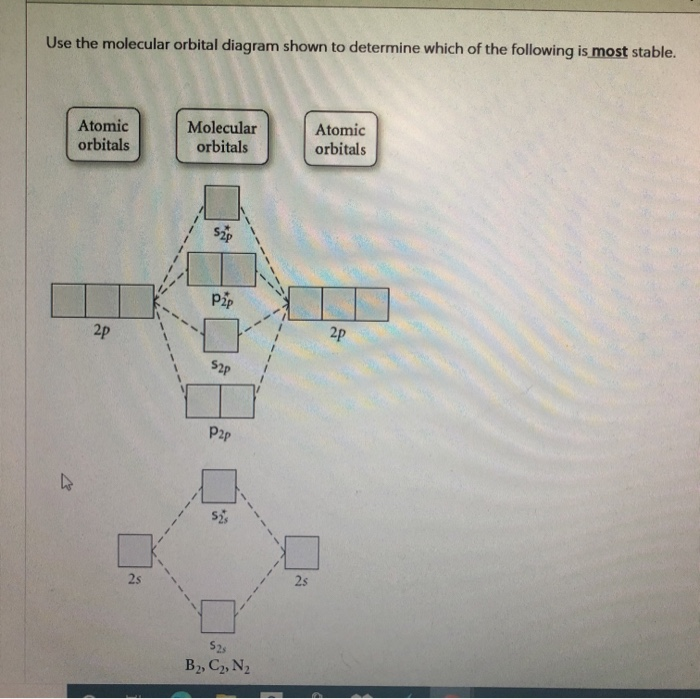



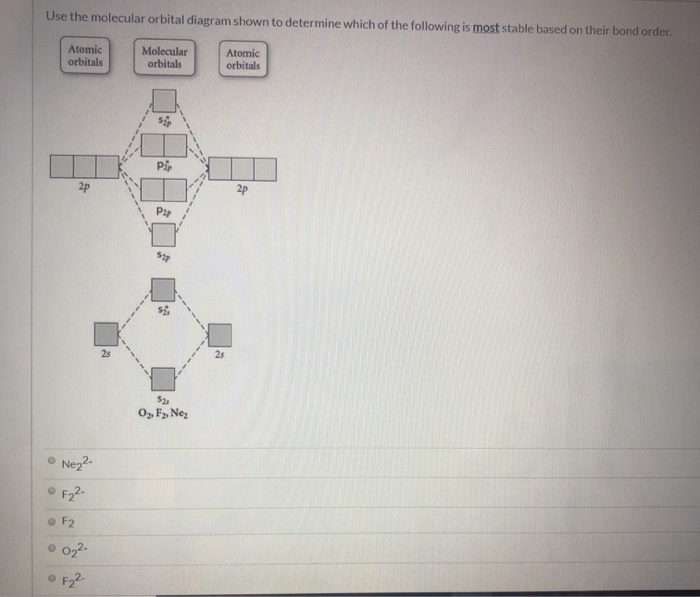
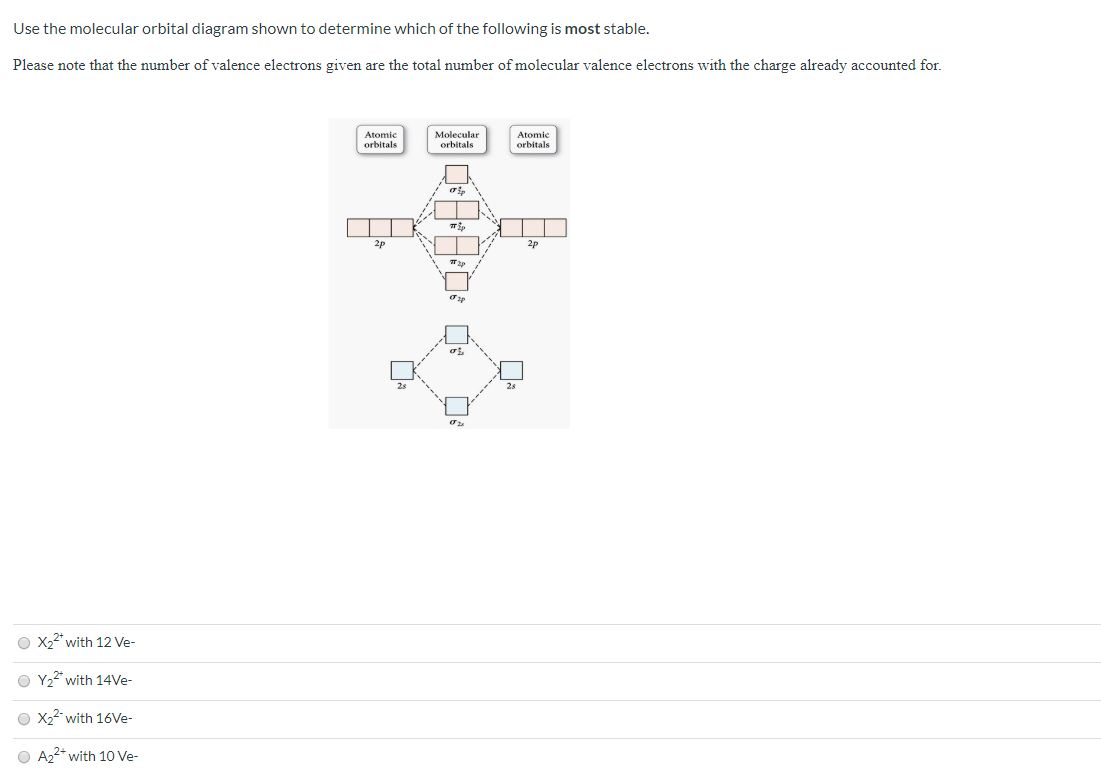
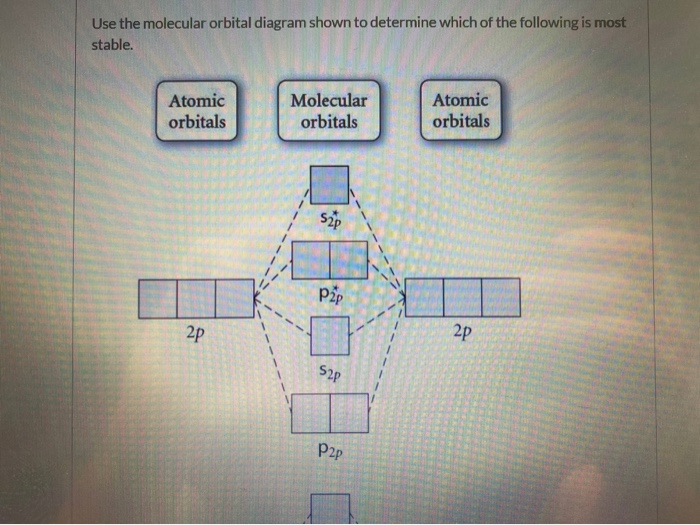
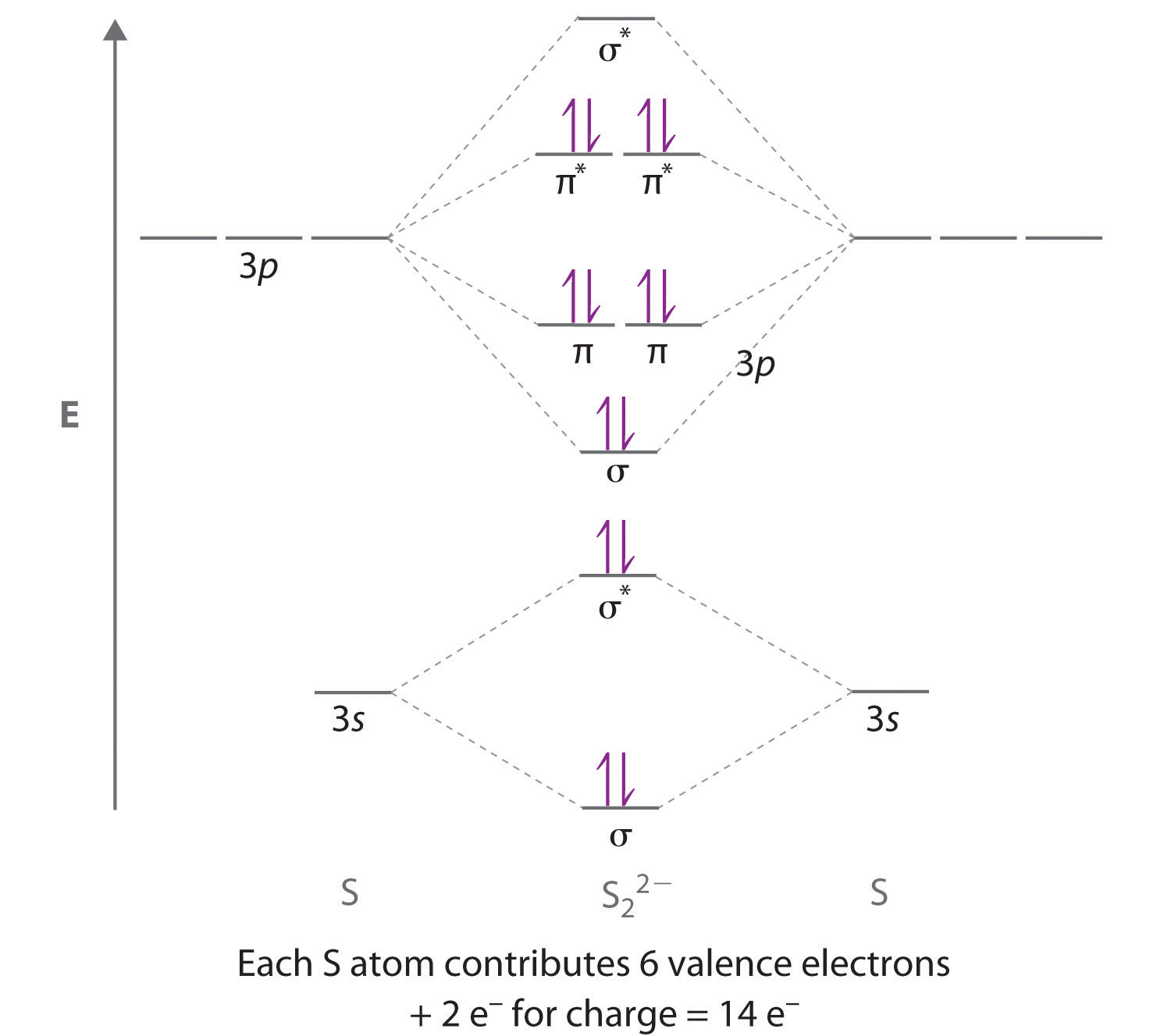



0 Response to "35 use the molecular orbital diagram shown to determine which of the following is most stable."
Post a Comment