35 bohr diagram of nitrogen
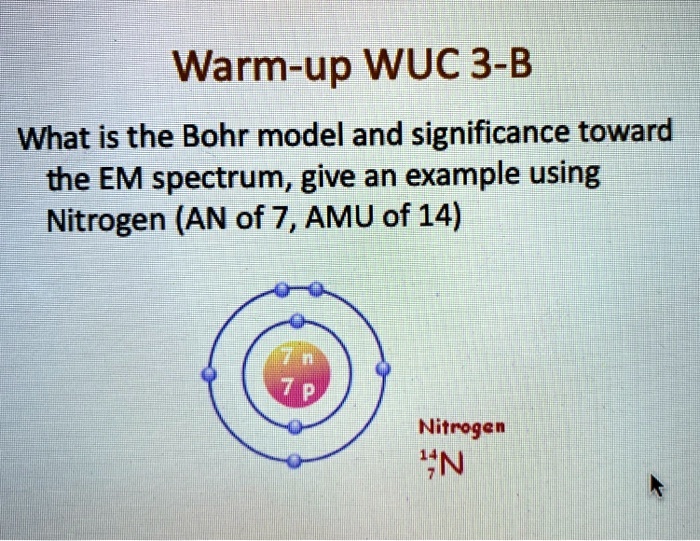
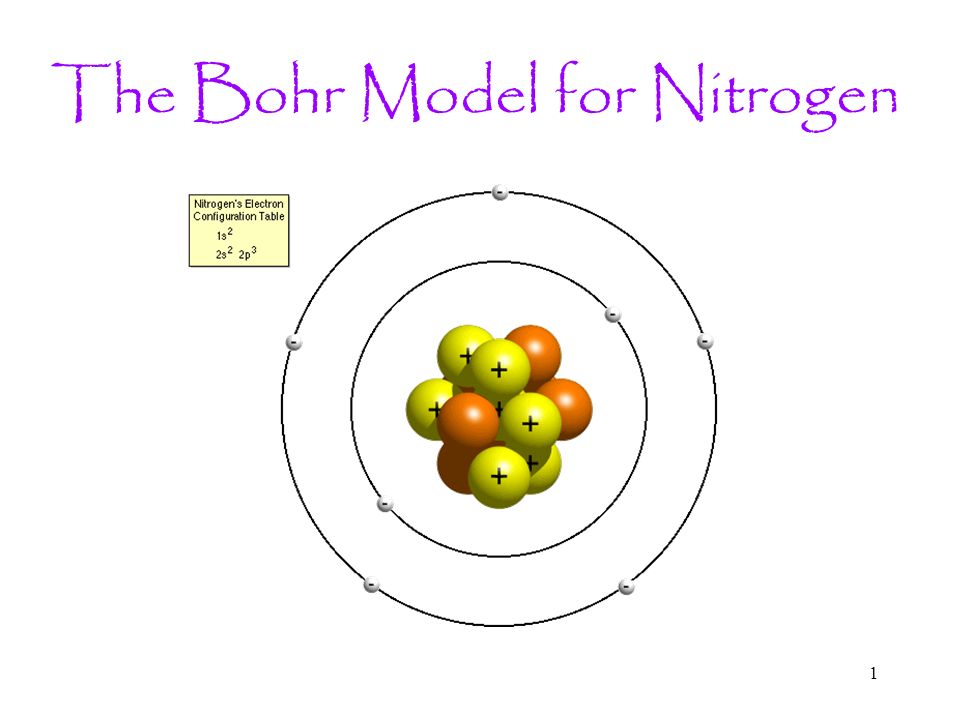
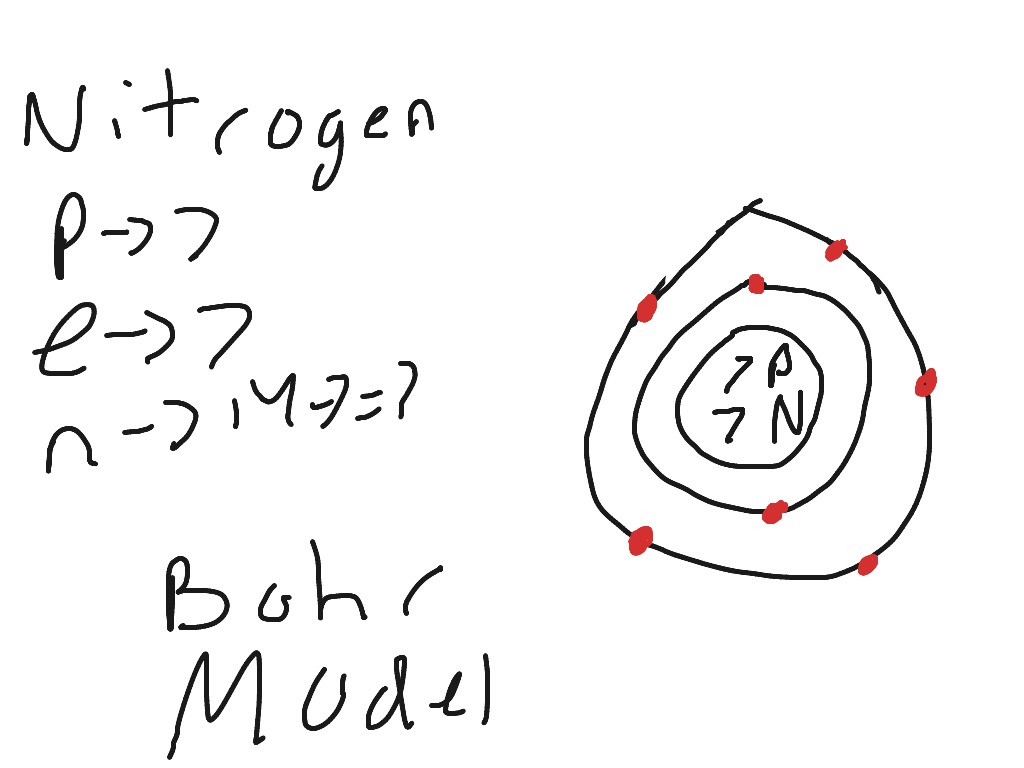
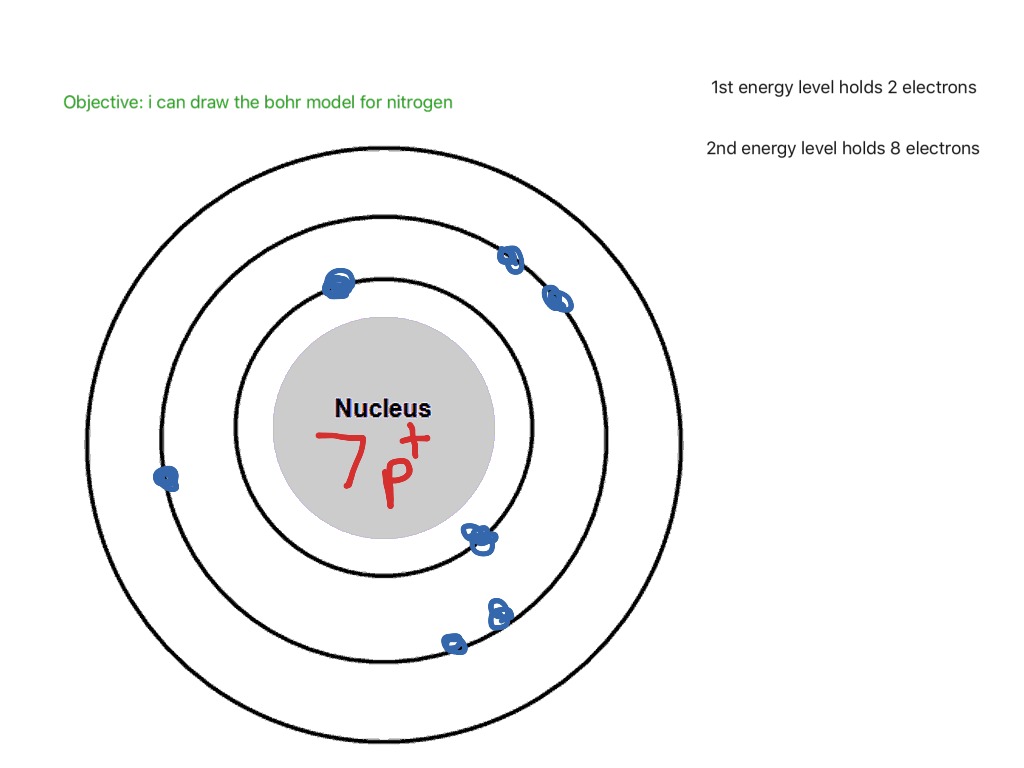
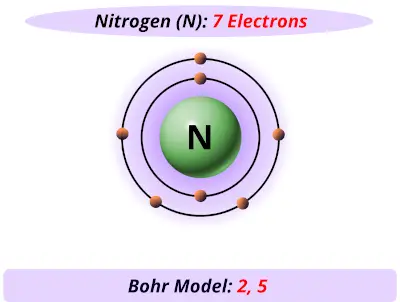
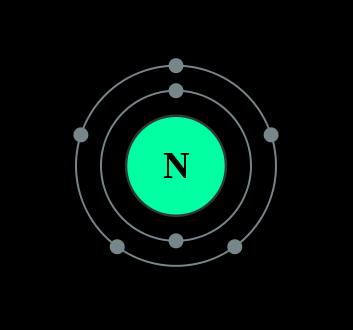
Bohr Diagrams So far we have used Bohr diagrams to show how may electrons an element has and how elements bond in ionic compounds. ... Nitrogen family g) Boron family h) Carbon family . 6 7. The diagram at the right shows the formation of LiH. a) How many valence electrons did the Li The Bohr Model of Nitrogen(N) has a nucleus that contains 7 neutrons and 7 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Nitrogen contains 5 electrons that also called valence electrons.
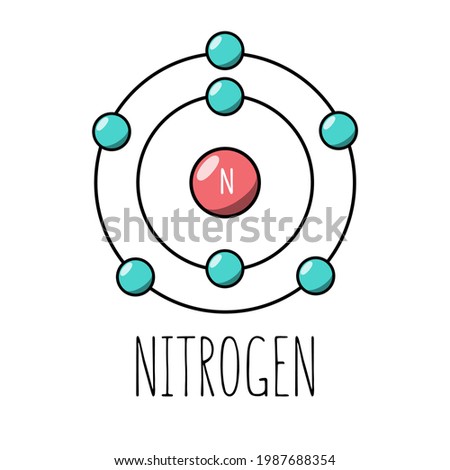
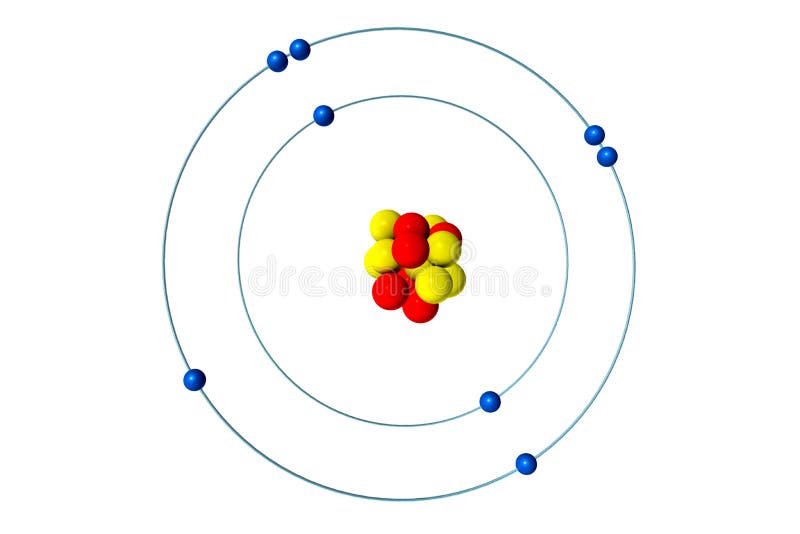
Bohr model for nitrogen by Allen Saunders - October 18, This is a collection of diagrams of atoms showing the numbers of protons, neutrons, This diagram shows the electron shell of a nitrogen atom. In the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.

Bohr diagram of nitrogen
Bohr Rutherford Diagram For Nitrogen. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. Bohr atomic model of a nitrogen atom. Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed () by the Danish. Bohr Ruthford Diagram helps. ... How many dots in the Lewis structure of nitrogen ion? There are 8 valence electrons in nitrogen ion, hence 8 dots. A Lewis dot structure is like a simplified Bohr-Rutherford model. The Lewis Dot diagram contains the element symbol with dots representing electrons. The only electrons shown are those on the outer energy level or valence electrons. The electrons are placed around the element symbol, one at a time, clockwise or counterclockwise, and then ...
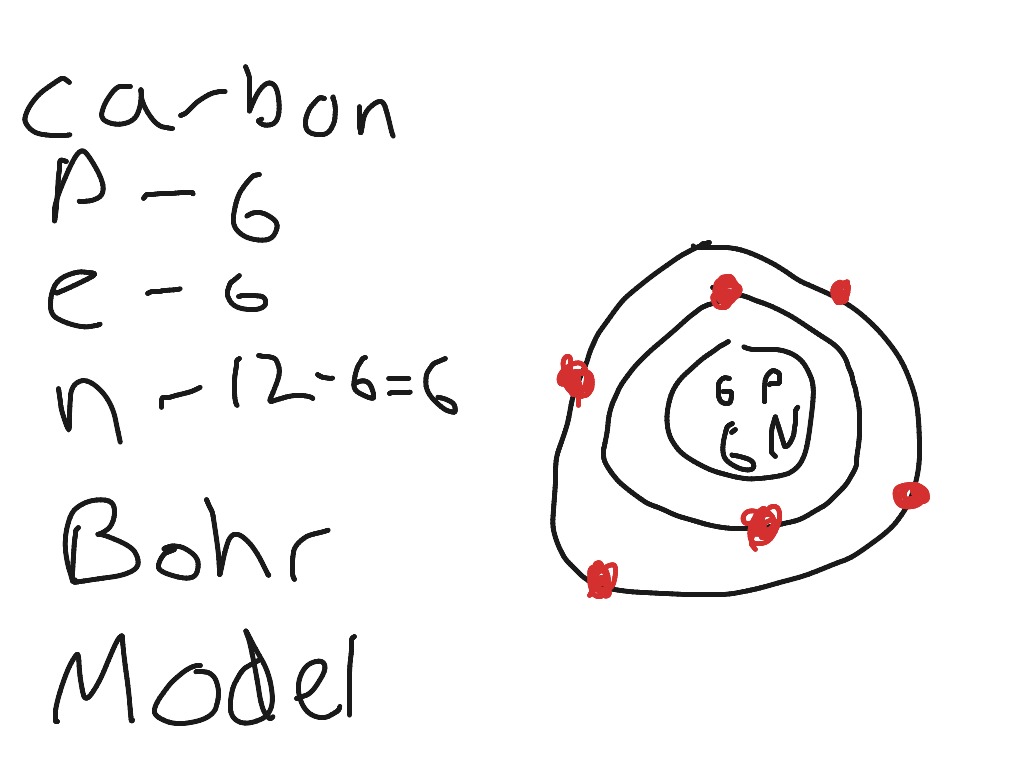
Bohr diagram of nitrogen. Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He Bohr Diagrams in terms of A Bohr diagram is a diagram that shows how many each shell surrounding the nucleus. Named in honour of , a Danish physicist who developed several models for showing the arrangement of electrons in atoms. There are three main background questions to explore before we start drawing Bohr diagrams. Nitrogen Atom Diagram Atom Model, Illustration, Diagram, Illustrations ... See the Electron Configuration Diagrams for Atoms of the Elements. What is the Bohr diagram for nitrogen? In the Bohr model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Two of the electrons are in the first energy level while the other five are in the second energy level. The number of available sub-shells increases as the energy level ...
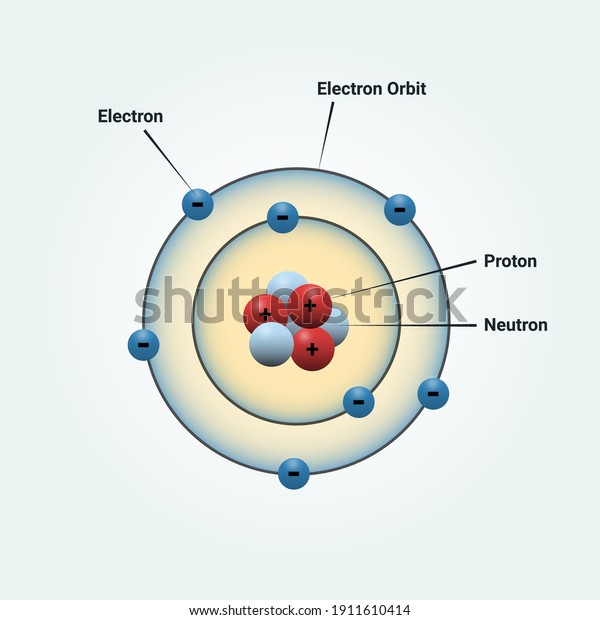
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons Nitrogen is a non-metal element that occurs most abundantly in the atmosphere, nitrogen gas (N2) comprises 78.1% of the volume of the Earth's air. A bohr rutherford diagram is used to show the numbers and locations of protons neutrons and electrons in an atom step 1 determine the number of protons and neutrons in the atom. Analyzing Dohr model diagrams Fill in the blanks beside each Bohr model diagram. The first one has been partially completed to help guide you. (a) (b) (c) (d) (a) (b) number of protons number of shells number of electrons number of valence electrons a nitrogen atom Bohr model of number of protons number of shells number of electrons Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for sale ...
Bohr diagram is very interesting and easy to draw. Let's see How to draw a Bohr diagram for an atom? To draw the Bohr model of an atom, we should follow 4 or 5 basic steps. Find the number of protons, electrons, and neutrons of an atom. Draw the nucleus of an atom. Write the number of protons and neutrons at the center of the nucleus. Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ... Molecular nitrogen (\(N_2\)) is not reactive at standard temperature and pressure and is a colorless and odorless gas. Figure \(\PageIndex{1}\) : A Bohr diagram of the nitrogen atom. Nitrogen is a non-metal element that occurs most abundantly in the atmosphere, nitrogen gas (N 2) comprises 78.1% of the volume of the Earth's air. It only ...
In the Bohr model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons. Two of the electrons are ...
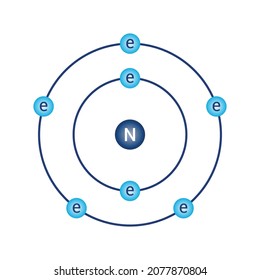
Bohr Diagram for Nitrogen. what is the bohr model of nitrogen the bohr model for nitrogen has a central nucleus with seven neutrons and seven protons a first energy ring with two electrons and a second energy ring with five electrons a more detailed version shows two electrons in the s sub shell of the second energy ring and three electrons in the p sub shell what does nitrogen bohr diagram ...
Bohr model for nitrogen by Allen Saunders - October 18, This is a collection of diagrams of atoms showing the numbers of protons, neutrons, This diagram shows the electron shell of a nitrogen atom. In the planetary model, a nitrogen atom has a central nucleus, composed of seven protons and seven neutrons, surrounded by seven electrons.
Once Bohr had worked out that the energy levels of hydrogen were quan- tized, i.e. In atomic physics, the Rutherford-Bohr model or Bohr model or Bohr diagram, presented by The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a.
Bohr Electron Configuration Drawing for Nitrogen Ion. Bohr Electron Configuration Drawing for Nitrogen Ion.
Therefore, number of neutrons in nitrogen = 7. Bohr Model 1: Draw a small circle, inside that circle, write p = 7 and n = 7 The first outer shell holds 2 electrons So draw, 2 electrons on the circle Then draw the second shell, which can contain 8 electrons However, you only require 5. This is how you draw a Bohr Diagram. Hope this helps !
1 answerThe Bohr model for nitrogen shows seven protons and neutrons in a central nucleus, with seven electrons orbiting around them. The atomic number of...
Name: Nitrogen Symbol: N Atomic Number: 7 Atomic Mass: 14.00674 amu Melting Point:-209.9 °C (63.250008 K, -345.81998 °F) Boiling Point:-195.8 °C (77.35 K, -320.44 °F) Number of Protons/Electrons: 7 Number of Neutrons: 7 Classification: Non-metal Crystal Structure: Hexagonal Density @ 293 K: 1.2506 g/cm 3 Color: colorless Atomic Structure
Nitrogen has 2 electrons in its first shell and 5 in its second.Check me out: http://www.chemistnate.com
In atomic physics, the Bohr model or Rutherford-Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.After the solar system Joseph Larmor model (1897), the cubical model (1902 ...
A bohr diagram is a simplified visual representation of an atom that was developed by danish physicist niels bohr in 1913. The electron configuration for nitrogen is 1s 2 2s 2 2p 3. How do you draw a bohr model of a nitrogen ion. A bohr model of a nitrogen atom could look like this. 12506 gcm 3 color.
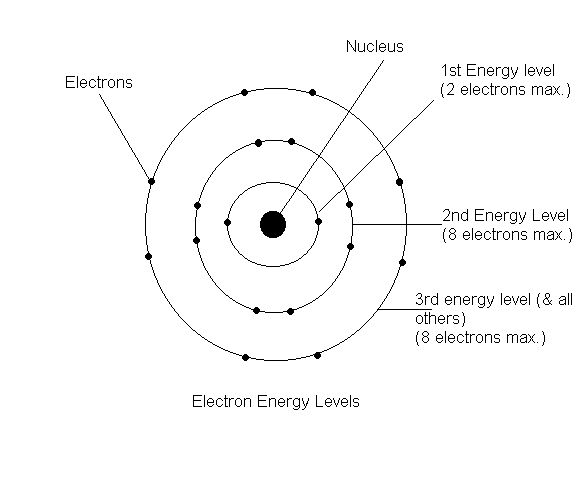
15 Aug 2020 — Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are ...Electron ShellsBohr DiagramsOrbitals in the Bohr modelBohr diagrams1 of 4Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the num...Continue on chem.libretexts.org »2 of 4Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, dependin...Continue on chem.libretexts.org »3 of 4Electrons fill orbit shells in a consistent order. Under standard conditions, atoms fill the inner shells (closer to the nucleus) first, often resulting in a variable number of electrons in the outerm...Continue on chem.libretexts.org »4 of 4Bohr diagrams indicate how many electrons fill each principal shell. G
Bohr won a Nobel Prize in Physics for his contributions to our understanding of the structure of atoms and how that is related to line spectra emissions. Key Concepts and Summary. Bohr incorporated Planck's and Einstein's quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra.
The Bohr model of hydrogen was the first model of atomic structure to correctly explain the radiation spectra of atomic hydrogen. It was preceded by the Rutherford nuclear model of the atom. In Rutherford's model, an atom consists of a positively charged point-like nucleus that contains almost the entire mass of the atom and of negative ...
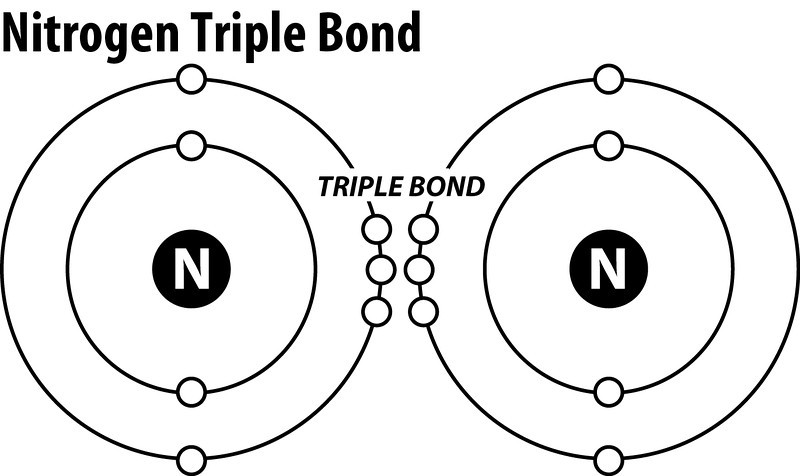
A Lewis dot structure is like a simplified Bohr-Rutherford model. The Lewis Dot diagram contains the element symbol with dots representing electrons. The only electrons shown are those on the outer energy level or valence electrons. The electrons are placed around the element symbol, one at a time, clockwise or counterclockwise, and then ...
Bohr Ruthford Diagram helps. ... How many dots in the Lewis structure of nitrogen ion? There are 8 valence electrons in nitrogen ion, hence 8 dots.
Bohr Rutherford Diagram For Nitrogen. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. Bohr atomic model of a nitrogen atom. Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed () by the Danish.















0 Response to "35 bohr diagram of nitrogen"
Post a Comment