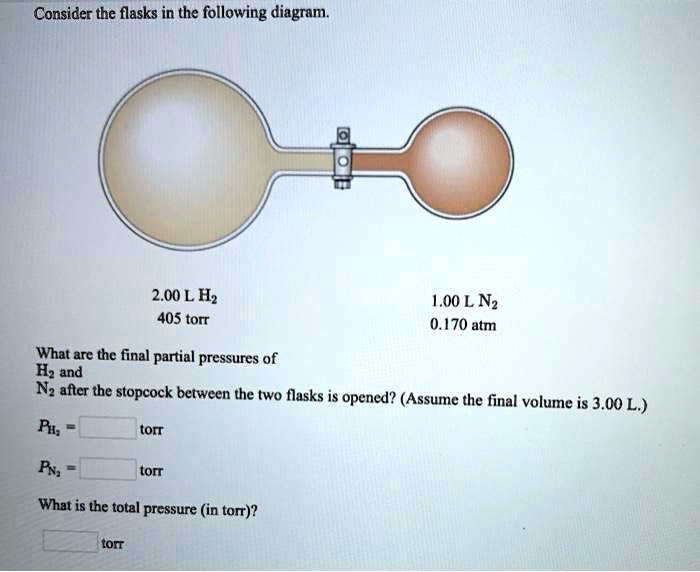
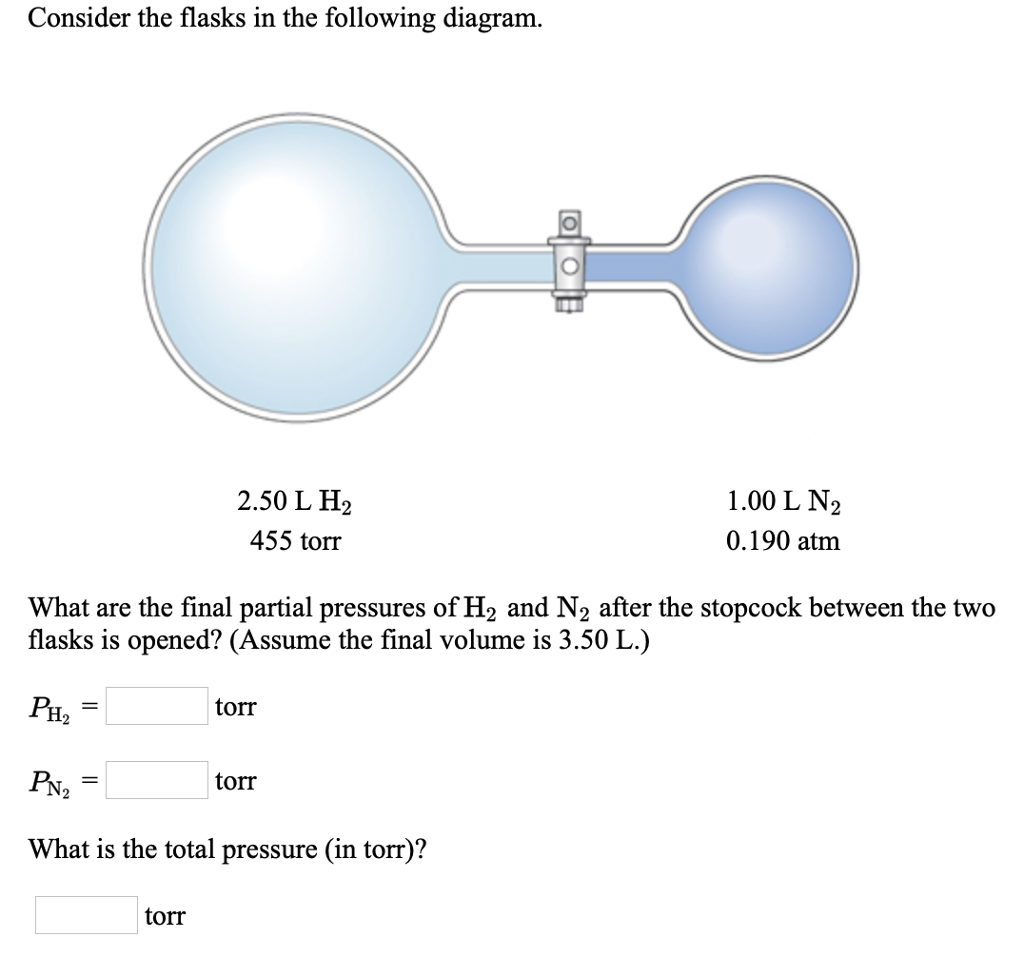
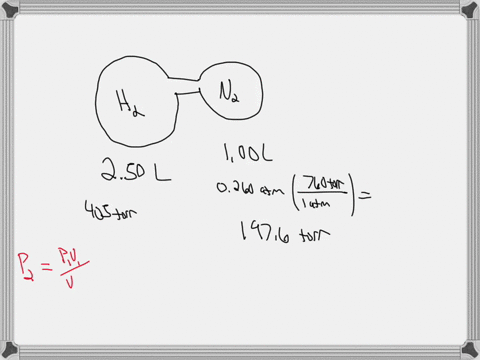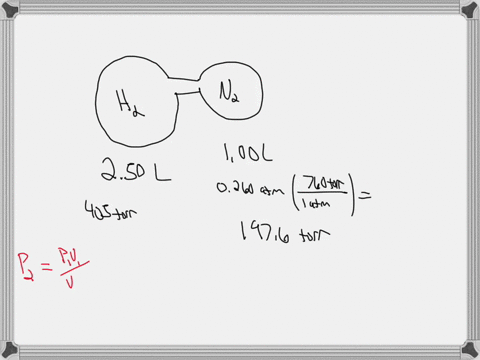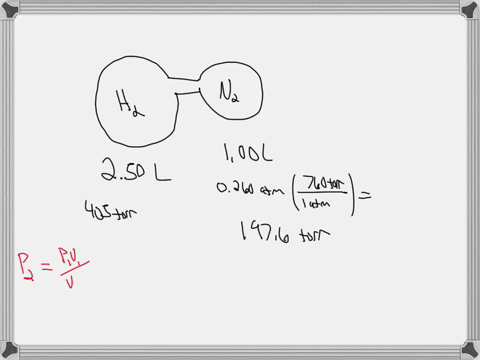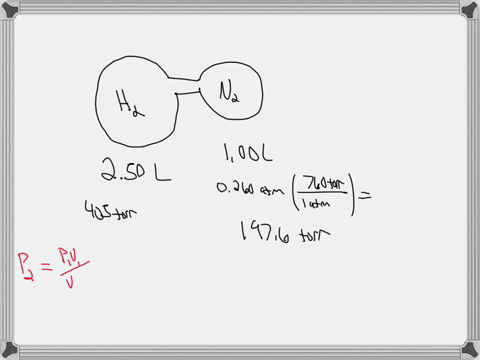
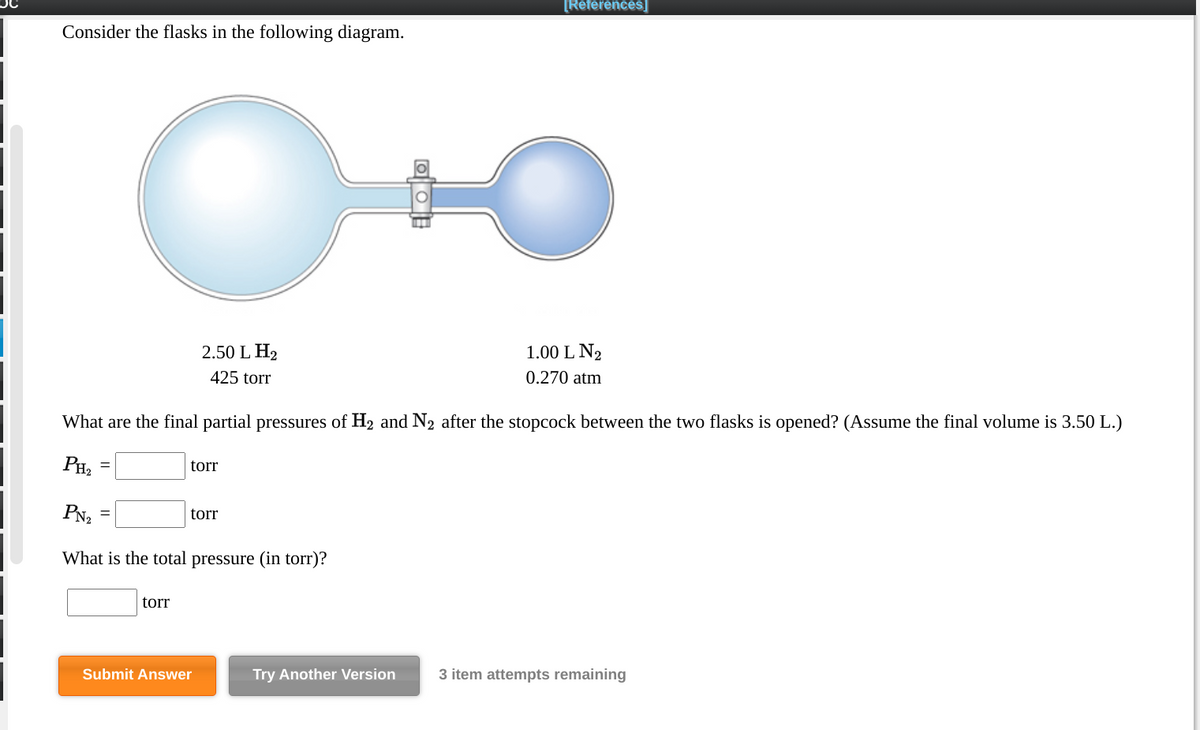
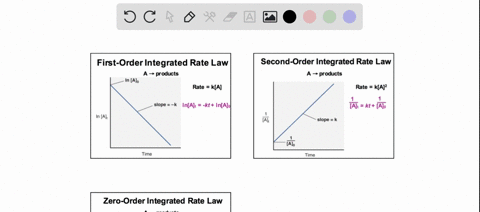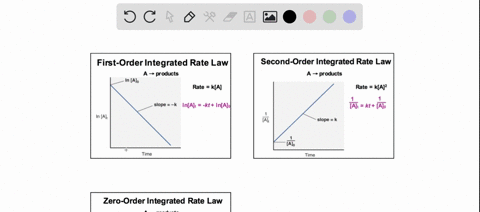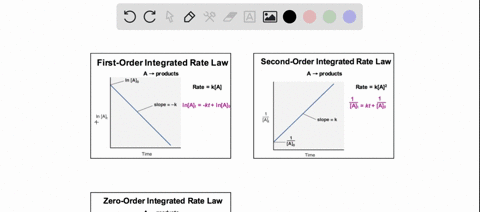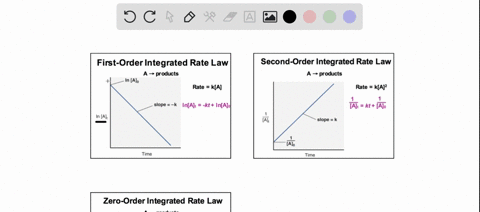
38 consider the flasks in the following diagram. what are the final partial pressures of h2 and n2
solucionario quimica de raymond chang 12 edicion - Academia.edu WebEnter the email address you signed up with and we'll email you a reset link. Bioprocess Engineering Principles-Pauline M. Doran WebEnter the email address you signed up with and we'll email you a reset link.
(PDF) SOLUTIONS MANUAL | Jisoo Kim - Academia.edu WebEnter the email address you signed up with and we'll email you a reset link.
Consider the flasks in the following diagram. what are the final partial pressures of h2 and n2
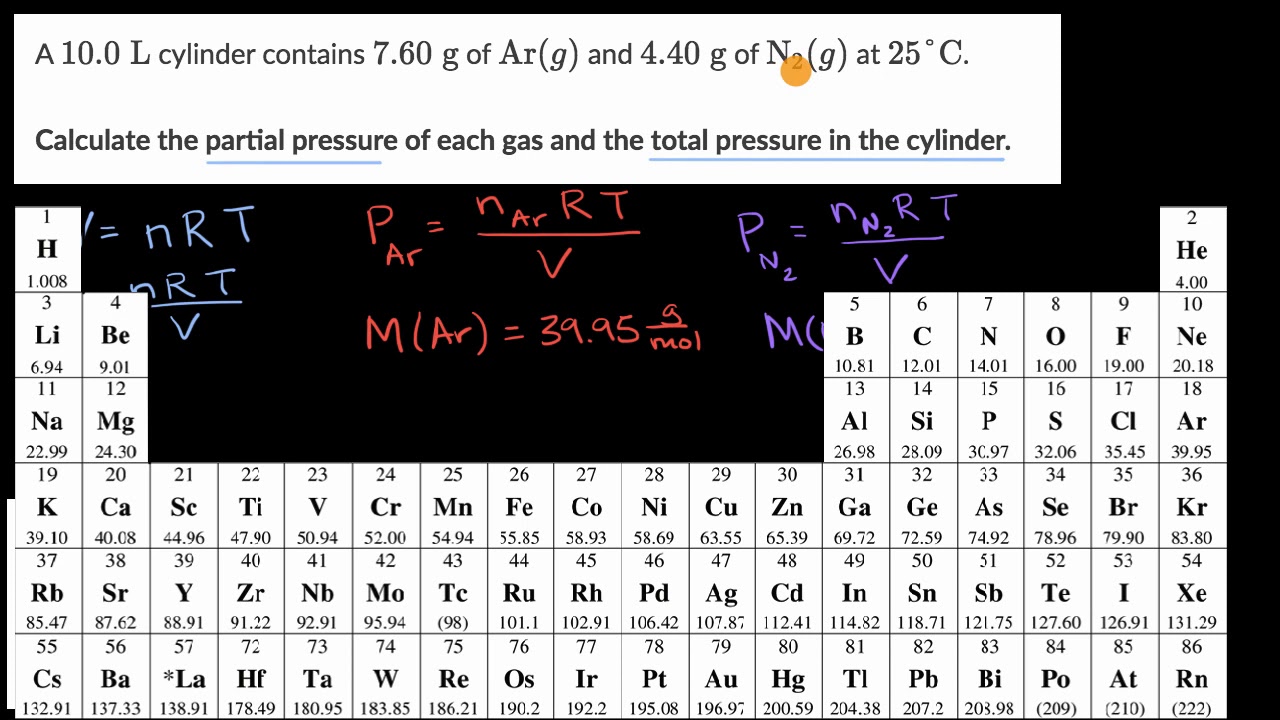
ap chem final unit 3 Flashcards | Quizlet WebA gas mixture at 0°C and 1.0atm contains 0.010mol of H2, 0.015mol of O2, and 0.025mol of N2. Assuming ideal behavior, what is the partial pressure of hydrogen gas (H2) in the mixture? ... Which statement describes the changes to the initial pressure of each gas and the final partial pressure of each gas in the mixture and also indicates the ... General Chemistry: Principles and Modern Applications (10th … WebAt this point, the temperature remains constant as the liquids vaporize. The mixed vapor condenses to produce two immiscible liquids; one liquid is essentially pure water and the other, pure cinnamaldehyde. The following vapor pressures of cinnamaldehyde are given: 1 mmHg at 76.1 °C; 5 mmHg at 105.8 °C; and 10 mmHg at 120.0 °C. Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS 5th ed … WebField tests for iodide, iodate, and iron: phase 2, final report. 1998 • Levente Diosady. Download Free PDF View PDF. Water quality monitoring: a practical guide to the design and implementation of freshwater quality studies and monitoring programmes. 1996 • Putsorn Chuntachorn. Download Free PDF View PDF. INDIAN PHARMACOPOEIA 2007 Volume 1 .
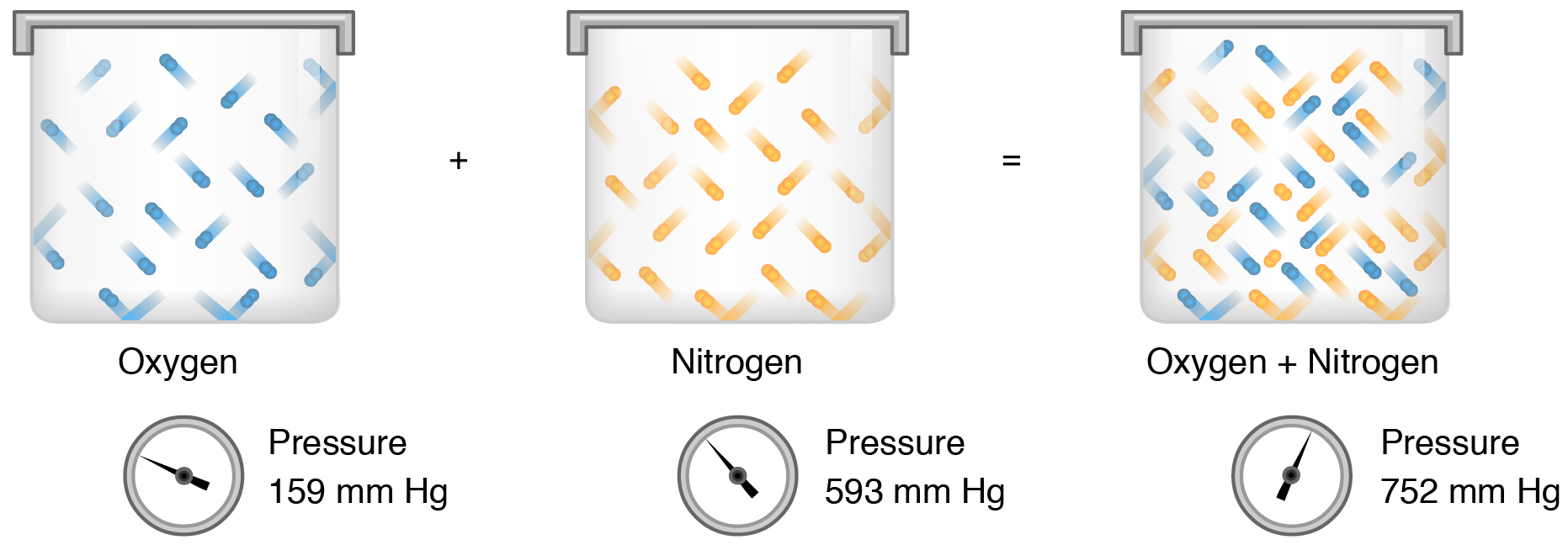
Consider the flasks in the following diagram. what are the final partial pressures of h2 and n2. Cambridge International AS and A Level Chemistry WebJC H2 Chemistry (1) Arijit Dasgupta. Download Free PDF View PDF. Problem-Solving Workbook with Solutions. Solomon Teshome (Northern Arizona University) and Raymond Chang, this success guide is written for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to ... (PDF) CHEMISTRY PDF | Janet Matthew - Academia.edu WebMetal-oxo and -peroxo species play key roles in catalytic oxidations using transition metals. This contribution addresses gas-phase studies of diatomic metal-oxo species and their properties which turn out to be of prime importance for … CHAPTER 1 CHEMISTRY: THE STUDY OF CHANGE Problem Categories … WebCheck: From the above conversion factors you can show that 1 m 3 = 1 × 10 3 L. Therefore, 7 m 3 would equal 7 × 10 3 L, which is close to the answer. − × = 3.1% Overwatch 2 reaches 25 million players, tripling Overwatch 1 daily ... WebOct 14, 2022 · Following a bumpy launch week that saw frequent server trouble and bloated player queues, Blizzard has announced that over 25 million Overwatch 2 players have logged on in its first 10 days."Sinc ...
Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS 5th ed … WebField tests for iodide, iodate, and iron: phase 2, final report. 1998 • Levente Diosady. Download Free PDF View PDF. Water quality monitoring: a practical guide to the design and implementation of freshwater quality studies and monitoring programmes. 1996 • Putsorn Chuntachorn. Download Free PDF View PDF. INDIAN PHARMACOPOEIA 2007 Volume 1 . General Chemistry: Principles and Modern Applications (10th … WebAt this point, the temperature remains constant as the liquids vaporize. The mixed vapor condenses to produce two immiscible liquids; one liquid is essentially pure water and the other, pure cinnamaldehyde. The following vapor pressures of cinnamaldehyde are given: 1 mmHg at 76.1 °C; 5 mmHg at 105.8 °C; and 10 mmHg at 120.0 °C. ap chem final unit 3 Flashcards | Quizlet WebA gas mixture at 0°C and 1.0atm contains 0.010mol of H2, 0.015mol of O2, and 0.025mol of N2. Assuming ideal behavior, what is the partial pressure of hydrogen gas (H2) in the mixture? ... Which statement describes the changes to the initial pressure of each gas and the final partial pressure of each gas in the mixture and also indicates the ...
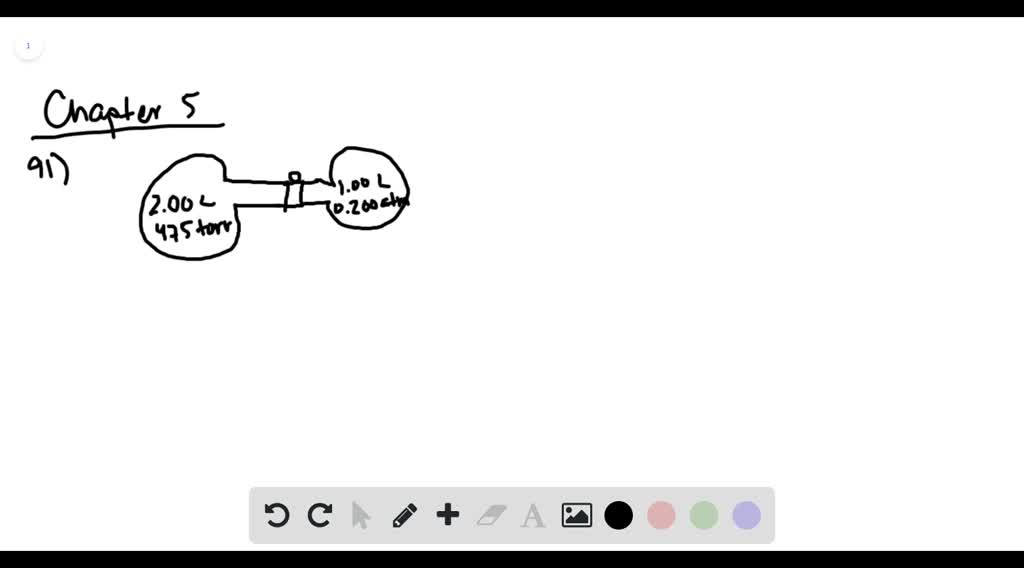
Consider the flasks in the following diagram. What are the final partial pressures of H2 and N2 after the stopcock between the two flasks is opened? (Assume the final volume is 3.00 L ) What is the ...

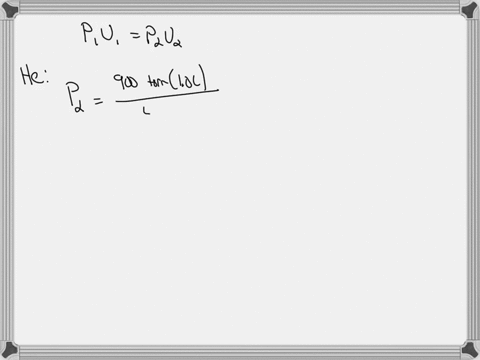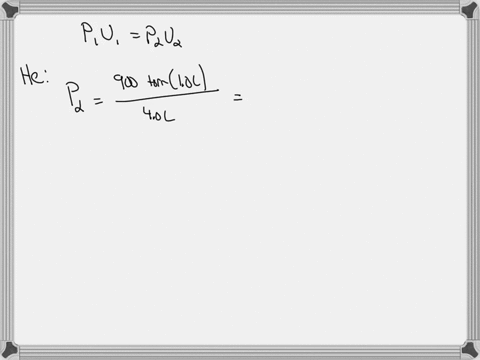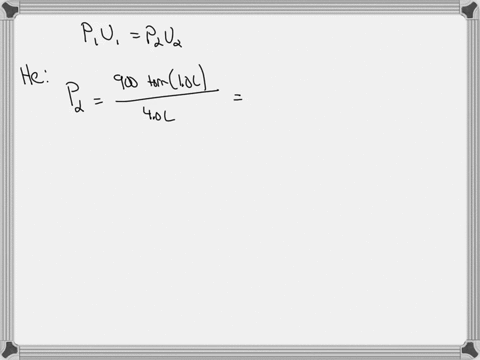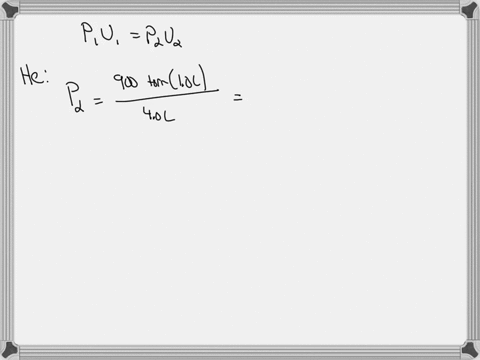











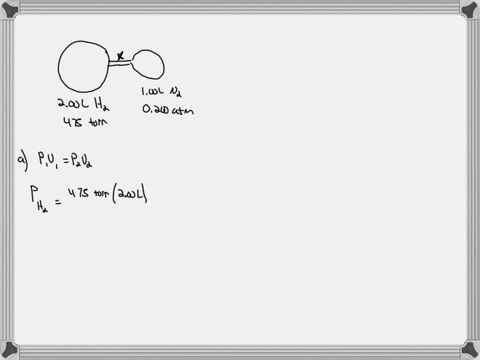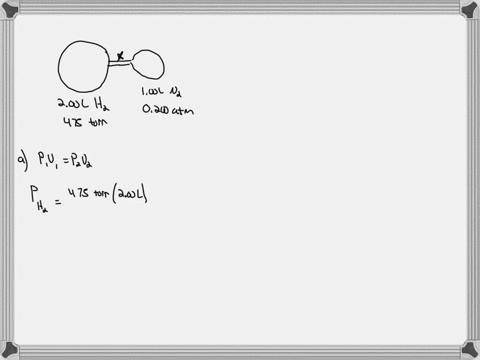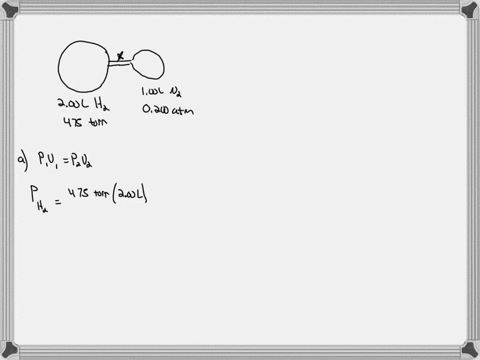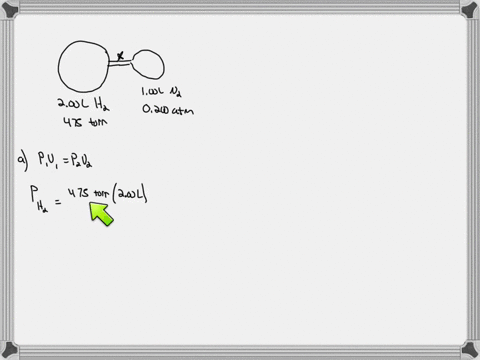





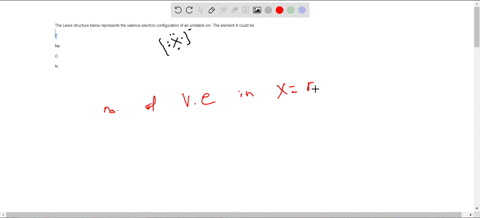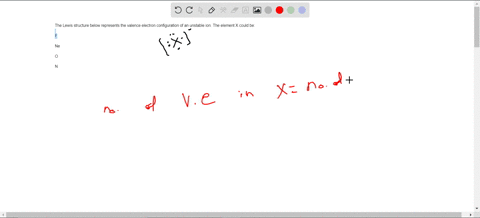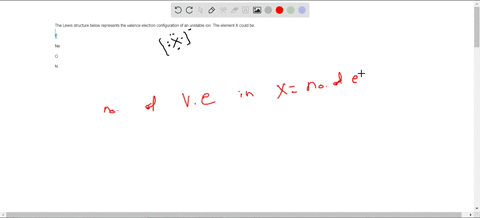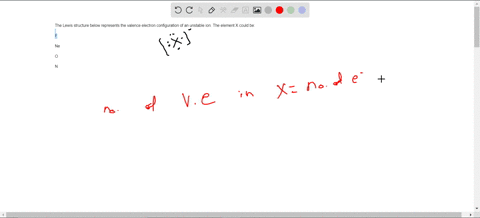


0 Response to "38 consider the flasks in the following diagram. what are the final partial pressures of h2 and n2"
Post a Comment