45 democritus atomic model diagram
Democritus - Stanford Encyclopedia of Philosophy Democritus' theory of perception depends on the claim that eidôla or images, thin layers of atoms, are constantly sloughed off from the surfaces of macroscopic bodies and carried through the air. Later atomists cite as evidence for this the gradual erosion of bodies over time. What does an atom look like? - WongChemistry Democritus. Dalton's. Model of Atom. Thomson's ... The First Atomic Theory ... c) Complete the orbital diagram for helium. 2. Carbon: a) atomic number: ...
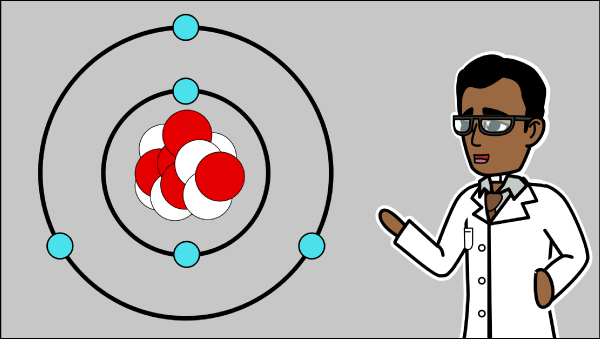
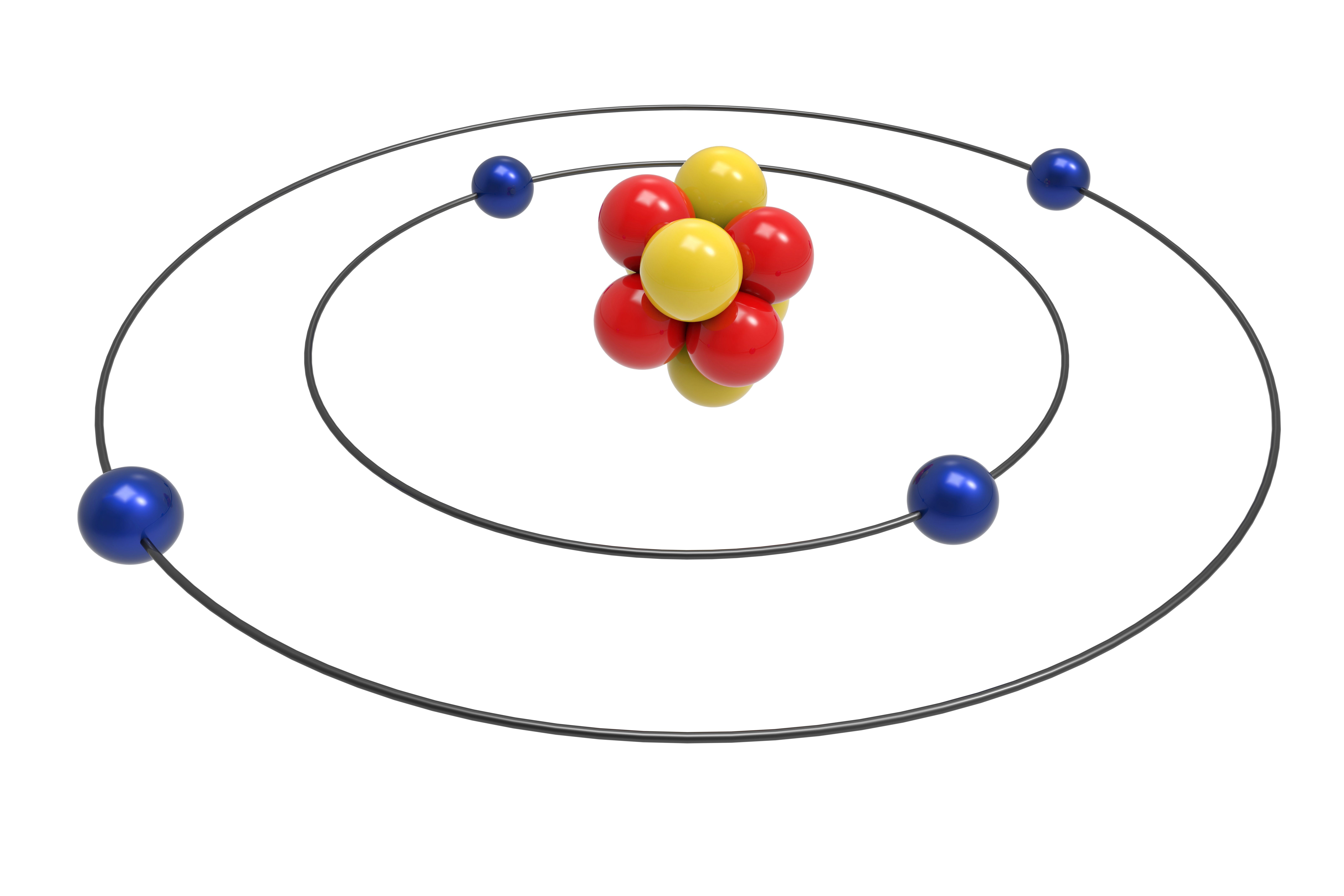
Atomic Models: Thomson's Atomic Model and Rutherford's Atomic Model Thomson's Atomic Model. In 1898, J. J. Thomson proposed the first of many atomic models to come. He proposed that an atom is shaped like a sphere with a radius of approximately 10-10 m, where the positive charge is uniformly distributed. The electrons are embedded in this sphere so as to give the most stable electrostatic arrangement.
Democritus atomic model diagram
5 Different Atomic Models- Theories, Diagram & Structure of Atom The word atom came from the Greek word Atomos which means indivisible. Atoms are made of electrons, Protons, and Neutrons. Protons and Neutrons reside in the nucleus of the atom and electrons orbit the nucleus. Atoms always have an equal number of protons and electrons and the number of neutrons and protons is usually the same as well. Development of atomic theory | Britannica In Democritus's philosophy, atoms existed not only for matter but also for such qualities as perception and the human soul. For example, sourness was caused by ... Atomic Model | Democritos, Dalton, Thompson, Rutherford, Nagaoka, Bohr Here are the different types of atomic model: Democritus, Dalton, Thompson, Rutherford, Nagaoka, Bohr, Sommerfeld, Schrodinger and Quantum Mechanics: 1. Democritus 2. Dalton 3. Thomson 4. Nagaoka 5. Rutherford 6. Bohr ... For ease of understanding, we will consider the simplest atoms to show some diagrams that reveal the basic points of the model:
Democritus atomic model diagram. What is Democritus Atom Model | fully explained - Technopython What is the democritus atom model? In democritus atom model, atoms exist not only in matter, but also in properties such as perception and the human soul. Differences in atomic shape and size determine different properties of matter. Changes in matter are the result of dissociation or combination of atoms as they move through the void. Democritus Atomic Theory | Biography & Facts of a Revolutionary Philosopher Democritus Atomic Model Essentially, the model was one of an inert solid that excluded other bodies from its volume, and whose interaction with other atoms was mechanical. The model worked with physical links, such as balls and sockets and hooks and eyes, to show in detail how connections happened between them. Difference Between Democritus and Dalton Atomic Theory Democritus atomic theory is the ancient theory that describes the nature of matter in terms of atoms. According to Democritus (99-55 BC), atoms are infinite in number and eternal. Figure 01: Democritus We cannot create them, and the composition of atoms in a substance determines the qualities of that substance. Developing models of atoms - Atomic structure - BBC Bitesize The plum pudding model The Geiger-Marsden experiment (1909 - 1911) Hans Geiger and Ernest Marsden tested the plum pudding model. They aimed beams of positively-charged particles at very thin gold ...
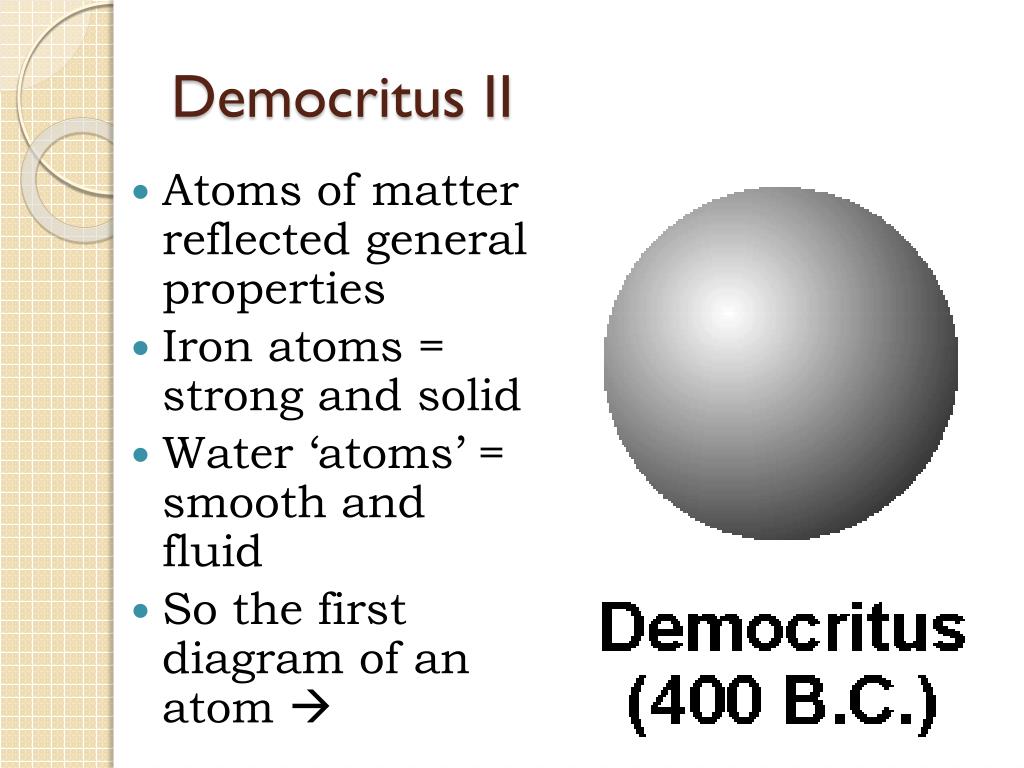
What is Democritus Atomic Theory? - History Flame The Democritus Atomic Theory said that everything in this world is composed of "Atoms". They are physically, but not geometrically, indivisible. Atoms are indestructible, there lies a space between them, they are infinite in numbers, they have always been and always will be in motion, and they vary in shape and size. Shape and Connectivity of Atoms Thomson atomic model | Description & Image | Britannica Thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by William Thomson (Lord Kelvin) and strongly supported by Sir Joseph John Thomson, who had discovered (1897) the electron, a negatively charged part of every atom. Though several alternative models were advanced in the 1900s by Kelvin and others, Thomson held that atoms are uniform ... Democritus - Biography, Facts and Pictures - Famous Scientists Lived c. 460 — c. 370 BC Democritus, the laughing philosopher, had ideas far in advance of his time. He is famous for his atomic theory featuring tiny particles always in motion interacting through collisions; his belief that the universe is governed entirely by natural, mechanistic laws rather than gods; his description of a universe Atomic theory, Democritus model, Atom - Pinterest During the classical period, Democritus came up with the atomic theory of matter which states that all things are made up of small spheres called atoms.
Definition & Different Atomic Models - StudySmarter The concept of the atom comes from a Greek philosopher named Democritus. He stated that all matter is made of indivisible particles called atoms surrounded by ... Atom Models - Democritus, Dalton, Thompson, Rutherford, Bohr 400 B.C. - Democritus thought matter could not be divided indefinitely. ... 1800 -Dalton proposed a modern atomic model ... Bohr - Rutherford diagrams. Atomic Theory Timeline - Coggle Atomic Theory Timeline - Coggle Diagram: Atomic Theory Timeline. ... Democritus thought that all atoms were created from the same material, however, ... What Is John Dalton's Atomic Model? - Universe Today the state that elements, in their purest state, consist of particles called atoms; that atoms of a specific element are all the same, down to the very last atom; that atoms of different elements...
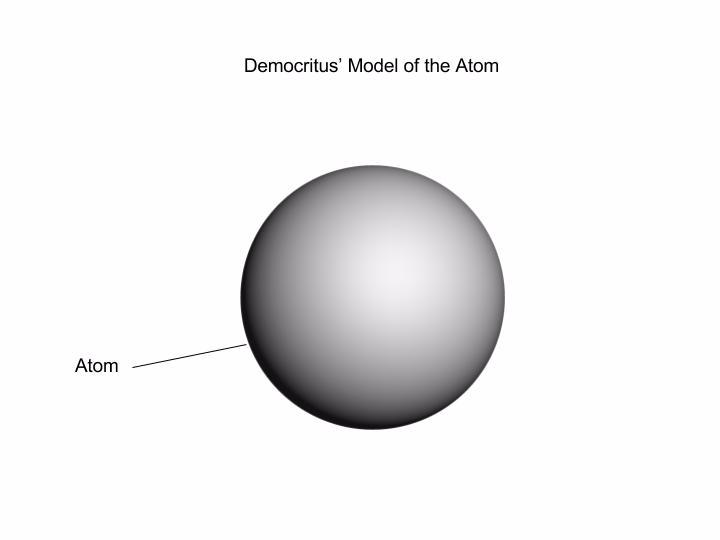
Democritus Atomic Model by Evelyn Medina - Prezi This was Democritus' atomic model. It was simply a round sphere with no electrons, protons, or neutrons. Democritus created the first atomic model so he contributed to helped people with understanding the idea of an atom, and helped other scientists further look into the science of the atom. Show full text.
The Atomic Model of Democritus: Structure and Motion He Democritus atomic model Is a theory that seeks to explain the structure and representation of atoms and their behavior from logical reasoning and philosophical principles. This model is extracted from the work Atomic Theory of the Universe Conceived by Leucipo but developed by the philosopher Democritus.
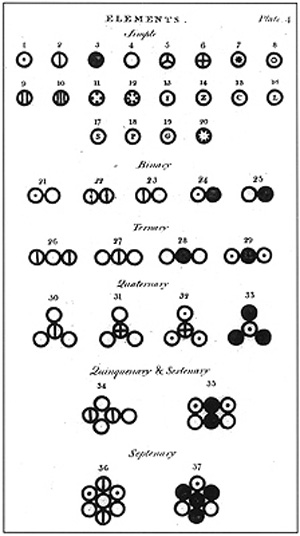
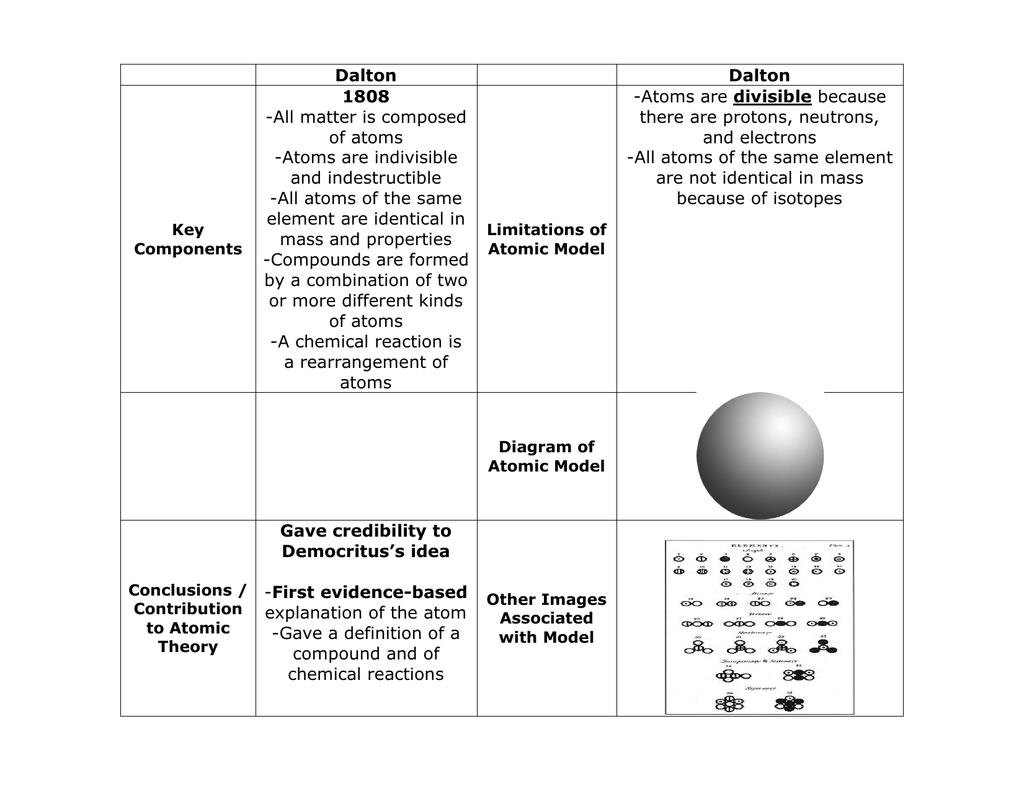
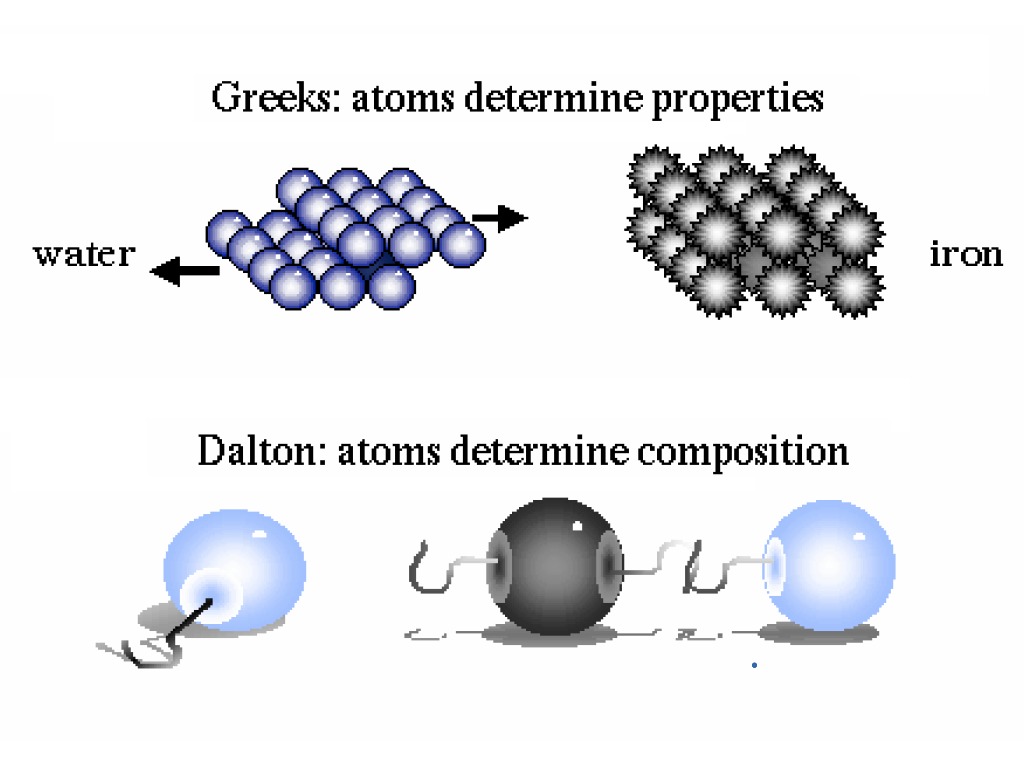
Dalton's Atomic Model | Brilliant Math & Science Wiki Based on all his observations, Dalton proposed his model of an atom. It is often referred to as the billiard ball model. He defined an atom to be a ball-like structure, as the concepts of atomic nucleus and electrons were unknown at the time. If you asked Dalton to draw the diagram of an atom, he would've simply drawn a circle!
Democritus - Wikipedia The theory of Democritus held that everything is composed of "atoms," which are physically, but not geometrically, indivisible; that between atoms, there lies empty space; that atoms are indestructible, and have always been and always will be in motion; that there is an infinite number of atoms and of kinds of atoms, which differ in shape and size.
Dalton's Model of the Atom and Early Atomic Theory - ThoughtCo Democritus recorded that Leucippus believed atoms to be small, indestructible bodies that could combine to change properties of matter. Aristotle believed elements each had their own special "essence," but he did not think the properties extended down to tiny, invisible particles.
Atomic Models: Definitions, Types & Demerits - Embibe Demerits of Dalton's Atomic Theory Atoms are now known to be made of subatomic particles- electrons, protons and neutrons. Isotopes are atoms of the same element with different mass number. Therefore, the assumption that all atoms have the same mass does not hold true. Allotropic form of the same elements with different properties was discovered.
Democritus Atomic Model | What was Democritus Atomic Theory? - Study.com Democritus contributed to the atomic model by imagining the first model of the atom. Democritus proposed that all things are composed of the atomos or the fundamental, indivisible...
What Was the Name of Democritus' Atomic Model? - Reference.com Democritus concluded, "Nothing exists except atoms and empty space." Before the Democritus model, ancient philosophers believed that everything was made up of either earth, wind, water or fire. Around 400 B.C., Democritus created this first model of the atom, believing that the atom was the smallest particle of matter.
Atom Democritus Dalton Thompson Rutherford Bohr Electron Cloud ... Dalton's Model – 1800's. • John Dalton came up with his atomic theory based on the results of his experiments. • Dalton published a paper about atoms which ...
5 Democritus Theory of Atoms - Structure - Model - AZ Chemistry In Democritus theory of atoms we can learn that the matter consists of atoms, the invisible parts, and the empty space or void. Democritus mentioned that atoms can not be destructed nor changed. He also stated that every atom is similar to each other which means that atom has no internal structure.
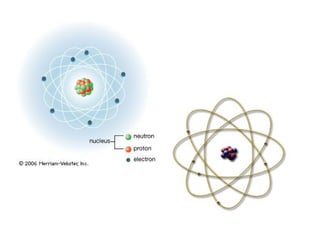
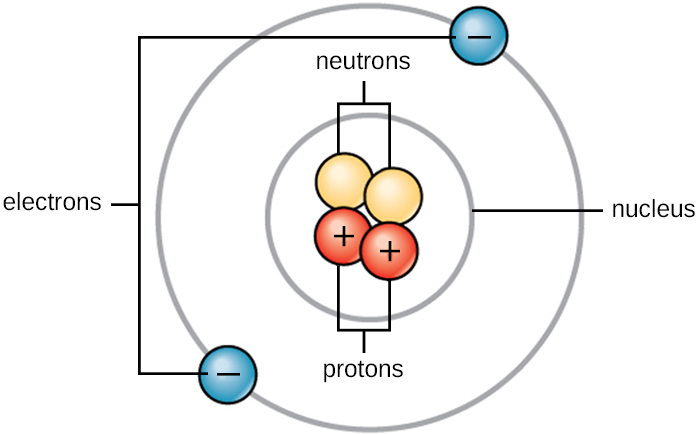
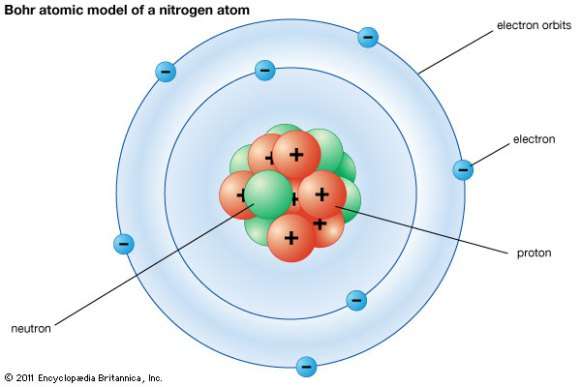
The Structure of an Atom Explained With a Labeled Diagram The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. This conclusion helped him propose 'Rutherford's Atomic Model'. According to his atom diagram, the atom has a small, positively charged nucleus in center. This nucleus carries the entire mass of the atom.
Democritus' Idea of the Atom ( Read ) | Chemistry | CK-12 Foundation Aug 3, 2016 ... A: The modern kinetic theory of matter is remarkably similar to Democritus' ideas about the motion of atoms. According to this theory, ...
Democritus | Biography & Facts | Britannica Democritus, (born c. 460 bce —died c. 370), ancient Greek philosopher, a central figure in the development of philosophical atomism and of the atomic theory of the universe. Knowledge of Democritus's life is largely limited to untrustworthy tradition.
Drawbacks of Democritus Atomic Theory - Origins and Impacts Atom theory according to Democritus: Atom is the smallest form of elements that form reality. It can't be seen by plain view because of the extremely small size. Atom has no quality, but it has quantity and mass number. Atom can be differed by its shape and portion. The number of atoms that formed reality is infinite.
The History of the Atom John Dalton was the first to adapt Democritus' theory into the first modern atomic model. JOHN DALTON'S ATOMIC MODEL: 1. All matter consists of tiny particles ...
4.1: Democritus' Idea of the Atom - Chemistry LibreTexts The atomists of the time (Democritus being one of the leading atomists) believed there were two realities that made up the physical world: atoms and void. There were an infinite number of atoms, but different types of atoms had different sizes and shapes. The void was the empty space in which the atoms moved and collided with one another.
Atomic Model | Democritos, Dalton, Thompson, Rutherford, Nagaoka, Bohr Here are the different types of atomic model: Democritus, Dalton, Thompson, Rutherford, Nagaoka, Bohr, Sommerfeld, Schrodinger and Quantum Mechanics: 1. Democritus 2. Dalton 3. Thomson 4. Nagaoka 5. Rutherford 6. Bohr ... For ease of understanding, we will consider the simplest atoms to show some diagrams that reveal the basic points of the model:
Development of atomic theory | Britannica In Democritus's philosophy, atoms existed not only for matter but also for such qualities as perception and the human soul. For example, sourness was caused by ...
5 Different Atomic Models- Theories, Diagram & Structure of Atom The word atom came from the Greek word Atomos which means indivisible. Atoms are made of electrons, Protons, and Neutrons. Protons and Neutrons reside in the nucleus of the atom and electrons orbit the nucleus. Atoms always have an equal number of protons and electrons and the number of neutrons and protons is usually the same as well.









![The Atom - Chemistry Grade 10 [CAPS] - OpenStax CNX](https://cnx.org/resources/935da4891bd882d3879c8bf7fd8a91c58c854260/CG10C3_001.png)




















0 Response to "45 democritus atomic model diagram"
Post a Comment