45 square planar orbital diagram
Tetrahedral vs. Square Planar Complexes - Chemistry LibreTexts May 10, 2021 · In square planar molecular geometry, a central atom is surrounded by constituent atoms, which form the corners of a square on the same plane. The square planar geometry is prevalent for transition metal complexes with d 8 configuration. The CFT diagram for square planar complexes can be derived from octahedral complexes yet the dx2-y2 level is ... molecular orbital diagram for square planar complexes - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
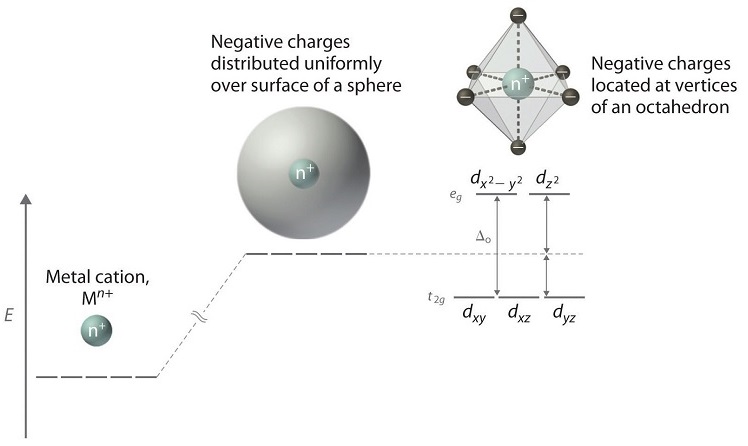
24.7: Crystal Field Theory - splitting patterns for octahedral ... We can use the d-orbital energy-level diagram in Figure 24.7. 1 to predict electronic structures and some of the properties of transition-metal complexes. We start with the Ti 3+ ion, which contains a single d electron, and proceed across the first row of the transition metals by adding a single electron at a time.
Square planar orbital diagram
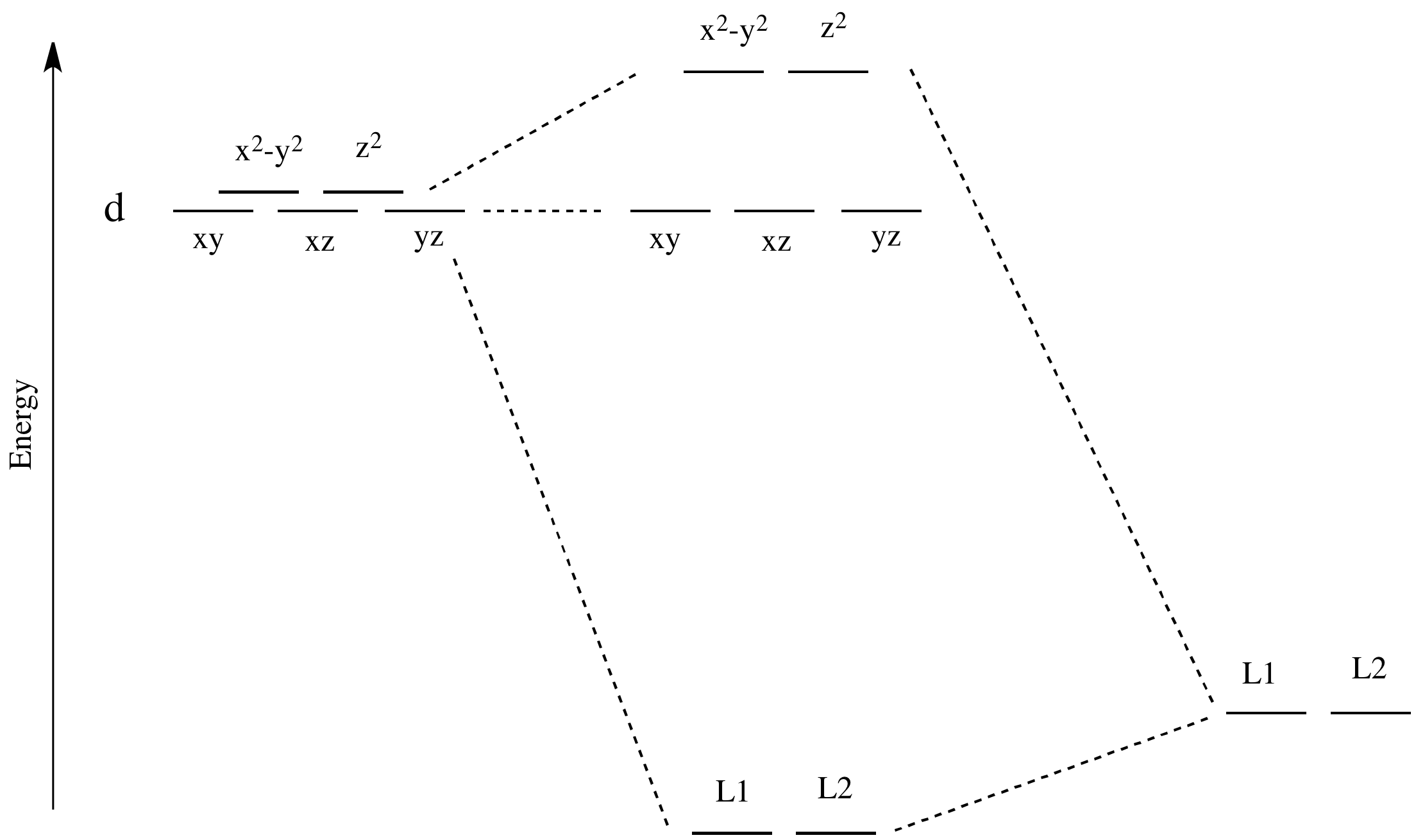
CHEM2P32 Lecture 11. Square and Tetrahedral Complexes - Brock University The orbital splitting diagram for square planar coordination can thus be derived from the octahedral diagram. As ligands move away along the z-axis, d-orbitals with a z-component will fall in energy. The d z2 orbital falls the most, as its electrons are concentrated in lobes along the z-axis. 10.3.5: Square-Planar Complexes - Chemistry LibreTexts Oct 25, 2022 ... That factor leads to square planar complexes generally adopting a low-spin configuration, which in this case means the lower orbitals are all ... Molecular Orbitals of Square-Planar Tetrahydrides | VIPEr This in-class activity walks students through the preparation of a molecular-orbital diagram for methane in a square-planar environment. The students generate ligand-group orbitals (LGOs) for the set of 4 H(1s) orbitals and then interact these with carbon, ultimately finding that such a geometry is strongly disfavored because it does not maximize H/C bonding and leaves a lone pair on C.
Square planar orbital diagram. Solved [10] Q18. (1) Draw the molecular orbital diagram for - Chegg (1) Draw the molecular orbital diagram for the square planar molecule XeF4. Show the mulliken symbol of orbitals. The ligand group orbitals of this molecule are shown below, from lowest energy to highest energy. LGO's 2&3 are degenerate. Only the radial p orbitals on F are involved in bonding, so don't bother to show the tangential p orbitals or Why is "Ni"("CN")_4^(2-) diamagnetic but "NiCl"_4^(2 ... - Socratic Their blank d -splitting diagrams within the realm of crystal field theory are: [Ni(CN)4]2−: The d orbitals fill with 8 electrons, then, with a low spin configuration. You can see that an even number of d orbitals will get filled ( dyz,dxz,dz2,dxy) with an even number of 3d electrons. This gives rise to a diamagnetic configuration, as expected. How do d orbitals split in a square planar crystal field? - NSN search There are four different energy levels for the square planar (from the highest energy level to the lowest energy level): d x2-y2, d xy, d z2, and both d xz and d yz. Figure 6: Splitting of the degenerate d-orbitals (without a ligand field) due to an square planar ligand field. How is d orbital splitting determined? PDF employees.oneonta.edu employees.oneonta.edu
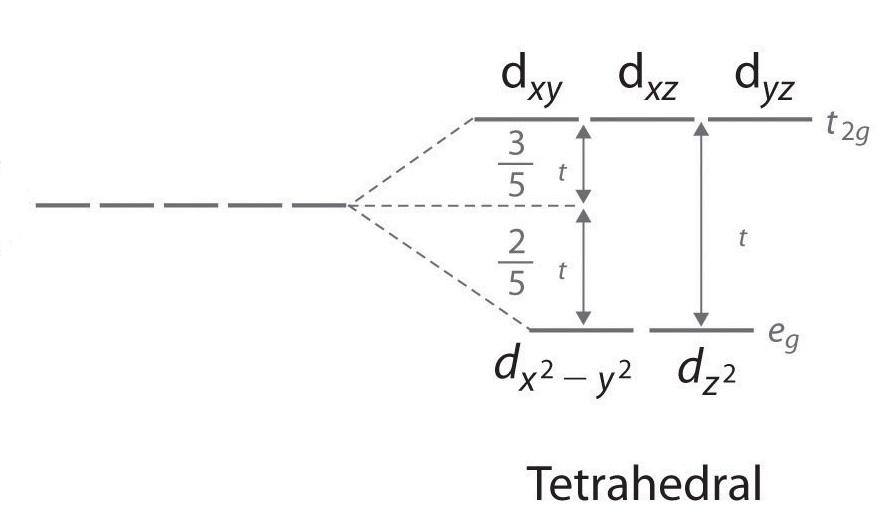
Molecular orbital theory for square planar complexes - YouTube Sep 5, 2021 ... Molecular orbital theory Square planar complexes Diagram with example explained Notes:- . PDF D-orbital splitting diagrams - University of California, Berkeley D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. Trigonal bipyramidal 4. Square pyramidal d z2x2-y d xy d yzxz 5. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d ... Why is PtCl4^2- square planar? | Socratic D ORBITAL SPLITTING FOR SQUARE PLANAR COMPLEXES The d orbitals look like this: We should recognize that since the ligands lie on the axes: The dx2−y2 orbitals experience the most repulsions. It is highest in energy. The dxy orbitals experience the second most. It is second highest in energy. Transition Metal d-Orbital Splitting Diagrams: An Updated Educational ... Here we provide a concise summary of the key features of orbital splitting diagrams for square planar complexes, which we propose may be used as an updated reference in chemical education. KEYWORDS: General Public Upper Division Undergraduate Inorganic Chemistry Cited By This article is cited by 18 publications.
Splitting of d-orbitals in square planar complexes of copper(II ... Splitting of d-orbitals in square planar complexes of copper(II), nickel(II) and cobalt(II) Author links open overlay panel Yuzo Nishida Sigeo Kida. Show more. Add to Mendeley. Share Cite. https ... Molecular Orbital Theory, Benjamin, New York (1964) 12. H. Yamatera. Bull. Chem. Soc. Jpn., 31 (1958), p. 95. CrossRef. 13. Square planar vs tetrahedral: Know the exact difference The square planar complexes form a four-tiered diagram in CFT. Tetrahedral complexes form a two-tiered crystal field diagram. The complexes forming square planar geometry has the electron configuration ending in d8. The configuration of the electrons in tetrahedral complexes can be from d0 or d10. 7 : Inorganic Chemistry-II - e-PG Pathshala form tetrahedral, square planar or pyramidal geometries. MOT for the tetra coordinated complexes can be utilized to construct the molecular orbital diagrams ... The Computational 2D Materials Database: high-throughput ... Sep 07, 2018 · The GLLBSC is an orbital-dependent exact exchange-based functional, which evaluates the fundamental gap as the sum of the Kohn–Sham gap and the xc-derivative discontinuity, . The method has been shown to yield excellent quasiparticle band gaps at very low computational cost for both bulk [ 102 , 103 ] and 2D semiconductors [ 36 ].
Square-planar, 16-electron complexes - Big Chemical Encyclopedia The 16-electron square planar complex is converted into an octahedral 18- electron complex. In Figure 2.14 we have depicted the oxidative addition of methyl iodide to Vaska s complex (L=phosphine). Iodide ions accelerate the reaction and addition of an anion to the metal is the first step in that instance [10]. [Pg.37]
PART 9(E): LIGAND FIELD THEORY (MO DIAGRAM SQUARE PLANAR ... - YouTube In this video, I have explained the detailed molecular orbital diagram for square planar complexes. Formation of sigma lgo and pi lgo have been discussed in detail.
Solved Label the d orbitals in the d-orbital energy diagram | Chegg.com Question: Label the d orbitals in the d-orbital energy diagram of a square-planar complex. — Answer Bank 0 I - - - xy NO yz xz A. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
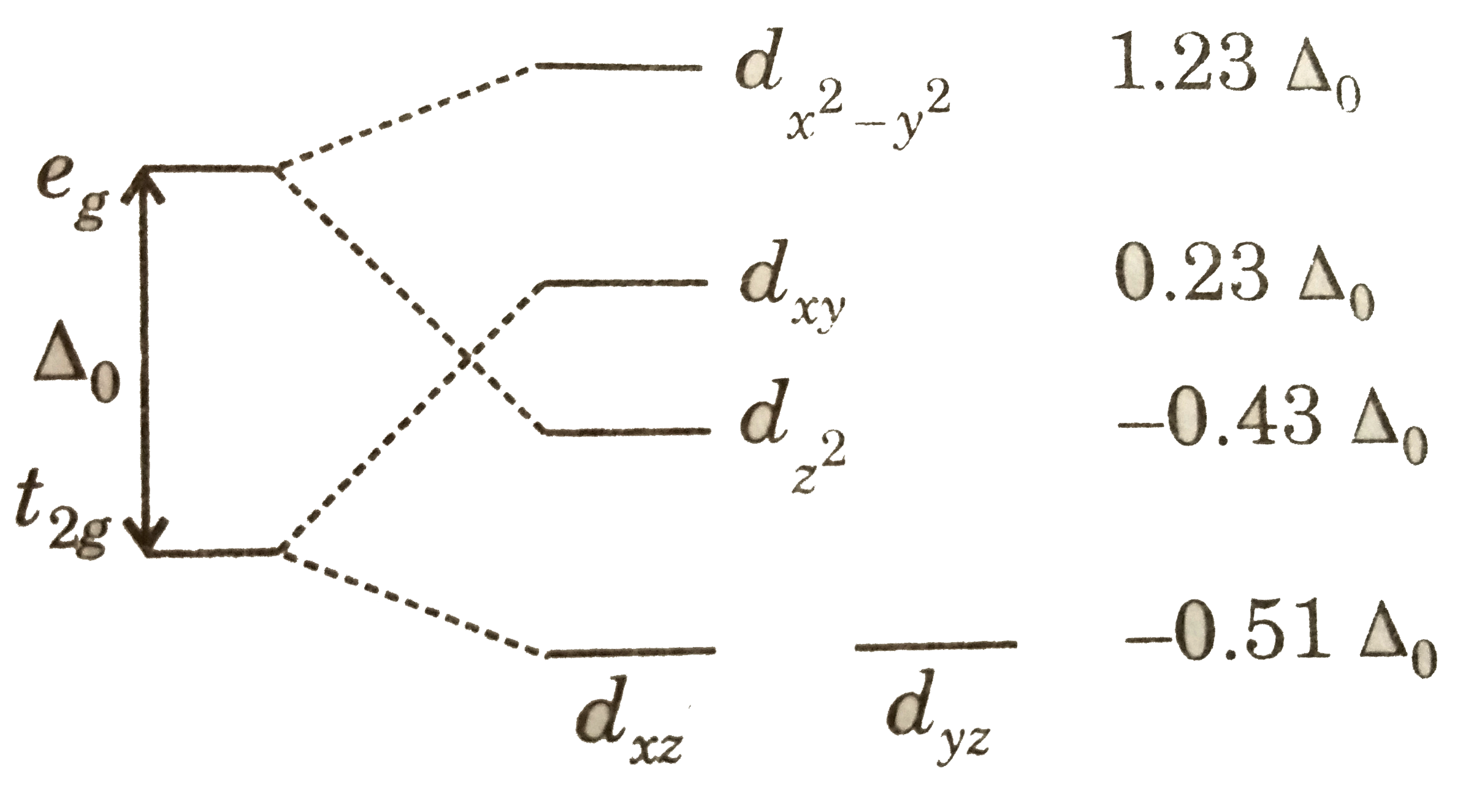
Why is [PdCl4]2- square planar whereas [NiCl4]2- is tetrahedral? The molecule [ P d C l X 4] X 2 − is diamagnetic, which indicates a square planar geometry as all eight d electrons are paired in the lower-energy orbitals. However, [ N i C l X 4] X 2 − is also d 8 but has two unpaired electrons, indicating a tetrahedral geometry. Why is [ P d C l X 4] X 2 − square planar if C l is not a strong-field ligand?
Molecular Orbital Theory – Octahedral, Tetrahedral or Square ... In octahedral complexes, the molecular orbitals created by the coordination of ... The molecular orbital energy level diagram for σ-bonding in square-planar ...
SPLITTING OF d - ORBITALS IN SQUARE PLANAR COMPLEXES Jul 20, 2020 ... This video is about SPLITTING OF d - ORBITALS IN SQUARE PLANAR COMPLEXES. ... Molecular Orbital Diagram For Pi Donor And Pi Acceptor Ligand ...
Could Call of Duty doom the Activision Blizzard deal? - Protocol Oct 14, 2022 · A MESSAGE FROM QUALCOMM Every great tech product that you rely on each day, from the smartphone in your pocket to your music streaming service and navigational system in the car, shares one important thing: part of its innovative design is protected by intellectual property (IP) laws.
Square planar molecular geometry - Wikipedia A general d-orbital splitting diagram for square planar (D 4h) transition metal complexes can be derived from the general octahedral (O h) splitting diagram, in which the d z 2 and the d x 2 −y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy, d xz and d yz orbitals.
square planar mo diagram Inorganic chemistry - Free Learn Diagram We have 9 Images about Geometry of Complex Ions - Chemistry LibreTexts like (a) Simplified MO diagram for a generic transition metal complex and, Molecular orbital energy level diagrams for: (a) a high-spin trigonal and also Geometry of Complex Ions - Chemistry LibreTexts. Read more: Geometry Of Complex Ions - Chemistry LibreTexts
MOLECULAR ORBITAL DIAGRAM FOR SQUARE PLANAR ... Jun 21, 2021 ... hellothis video class explains MOLECULAR ORBITAL DIAGRAM FOR SQUARE PLANAR COMPLEXES.
Three-body problem - Wikipedia A quantum-mechanical analogue of the gravitational three-body problem in classical mechanics is the helium atom, in which a helium nucleus and two electrons interact according to the inverse-square Coulomb interaction. Like the gravitational three-body problem, the helium atom cannot be solved exactly.
Molecular Structure & Bonding - Michigan State University Here, the correlation diagram correctly accounts for the paramagnetic character of this simple diatomic compound. Likewise, the orbital correlation diagram for methane provides another example of the difference in electron density predicted by molecular orbital calculations from that of the localized bond model. Click on the compound names for ...
Crystal field theory - Wikipedia If the splitting of the d-orbitals in an octahedral field is Δ oct, the three t 2g orbitals are stabilized relative to the barycenter by 2 / 5 Δ oct, and the e g orbitals are destabilized by 3 / 5 Δ oct.As examples, consider the two d 5 configurations shown further up the page. The low-spin (top) example has five electrons in the t 2g orbitals, so the total CFSE is 5 x 2 / 5 Δ oct = 2Δ oct.
Tetrahedral and Square Planar Complexes - Course Hero The CFT diagram for tetrahedral complexes has d x2−y2 and d z2 orbitals equally low in energy because they are between the ligand axis and experience little repulsion. In square planar molecular geometry, a central atom is surrounded by constituent atoms, which form the corners of a square on the same plane.
Earth - Wikipedia Earth's average orbital distance is about 150 million km (93 million mi), which is the basis for the Astronomical Unit and is equal to roughly 8.3 light minutes or 380 times Earth's distance to the Moon. Earth orbits the Sun every 365.2564 mean solar days, or one sidereal year. With an apparent movement of the Sun in Earth's sky at a rate of ...
Square planar molecular geometry | Detailed Pedia A general d-orbital splitting diagram for square planar (D 4h) transition metal complexes can be derived from the general octahedral (O h) splitting diagram, in which the d z 2 and the d x 2 −y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy, d xz and d yz orbitals. When the two axial ligands are removed to generate a square planar geometry, the d z 2 orbital ...
Square Planar Bond Angle The square planar, tetrahedral arrangement of molecules. ... The overall octahedral (Oh) separating model can be used to deduce an overall d-orbital dividing diagram for rectangular shape planar (D4h) metal oxides condos, wherein the dz3 and dx2y2 vibrational modes are depraved and have structure than the perverted set of the dsy, dky, and dsz ...
PDF Coordination Chemistry II: Jahn-Teller, Square Planar Complexes ... Jahn-Teller, Square Planar Complexes, Orbital Overlap Method, and Electron Counting Chapter 10 and Section 13.3 Monday, November 30, 2015. ... Square Planar Complexes Consider a CFT diagram of a tetragonal elongation taken to its extreme: tetragonal elongation removal of z ligands eg t2g b2g dxydxzdyz eg dz2 dx2-y2
Molecular Orbital Theory - Octahedral, Tetrahedral or Square Planar ... Zoom 100% Download "Molecular Orbital Theory - Octahedral, Tetrahedral or Square Planar Complexes" ATOICV1-7-2-Molecular-Orbital-Theory-Octahedral-Tetrahedral-or-Square-Planar-Complexes.pdf - Downloaded 293 times - 834 KB Share this article/info with your classmates/friends and help them to succeed in their exams.
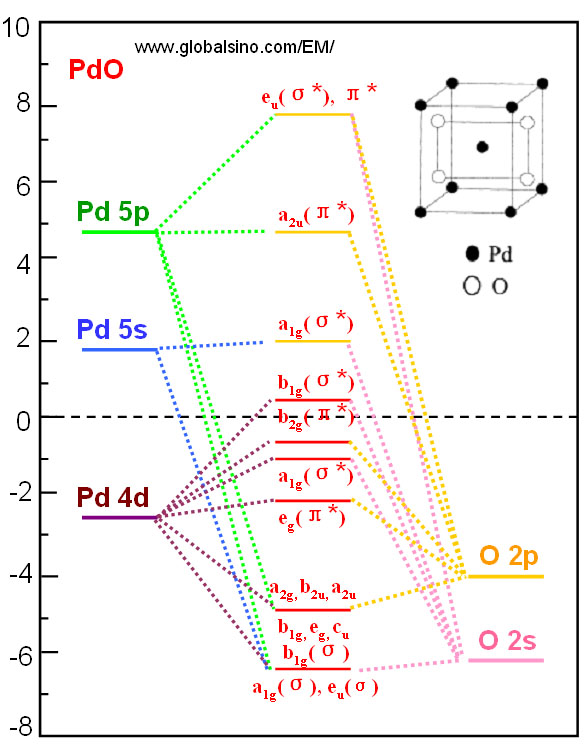
Frontier Orbital - an overview | ScienceDirect Topics the resultant frontier molecular orbitals are illustrated in figure 18. 142,145 for m (co) 2 there are four low lying levels of a 1, b 1, a 2 and a 1 symmetry consistent with the strong preference for d8 configurations for square planar geometries, and two higher lying levels of b 2 and a 1 symmetry which strongly resemble the frontier molecular …
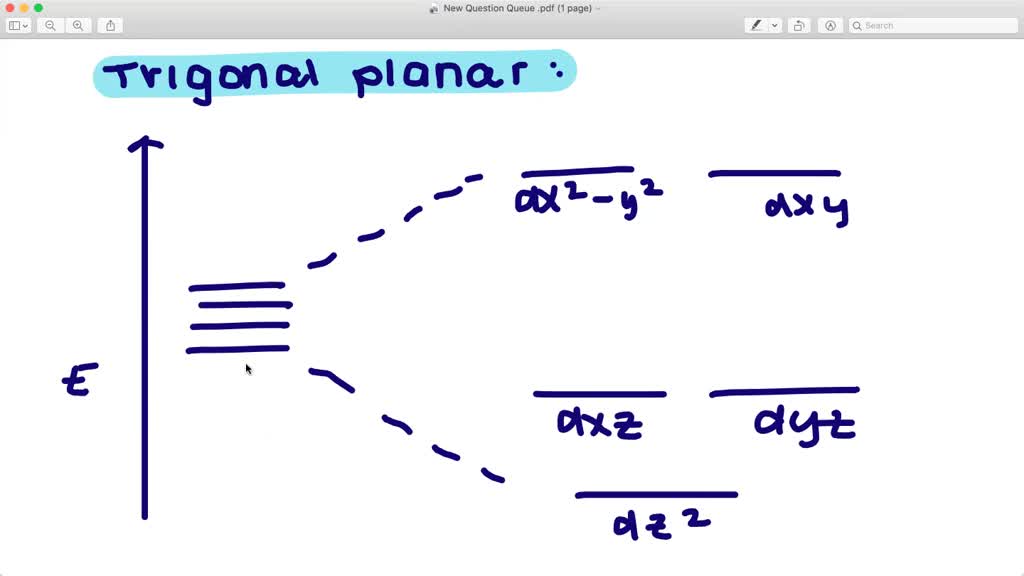
Qualitatively draw the crystal field splitting of the d orbitals in a trigonal planar complex ion. (Let the z axis be perpendicular to the plane of the complex.)
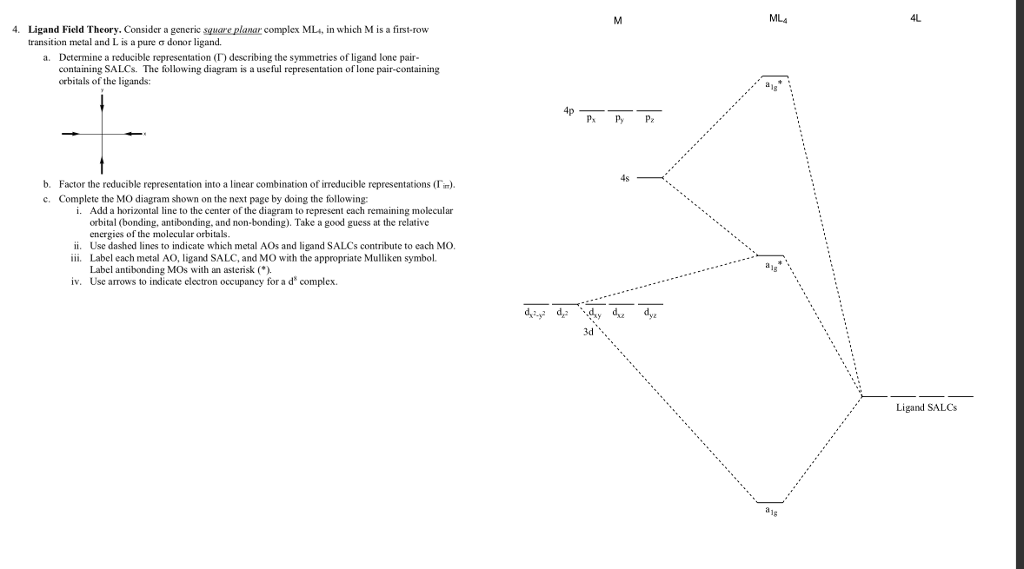
Molecular orbitals square planar complex - Big Chemical Encyclopedia An energy diagramfor the molecular orbitalsof a square-planar moleculeof formula ML4 (L = ligand that can function as both a donor and v acceptor) is shown in Figure 13-11. [Pg.465] FIGURE 13-11 Molecular Orbital Energy Levelsfor a Square-PlanarComplex. [Pg.466] Figure 1.
How can I build the molecular orbital diagram of the complex ... The next step is to start drawing an orbital diagram. The five d-orbitals should initially be on one level corresponding to the ionisation energy of nickel(II). The virtual 4s and 4p orbitals somewhat higher. The π-type ligand group orbital should be on a level corresponding to the ionisation energy of ammonia, the σ-type ones slightly below.
Molecular Orbitals of Square-Planar Tetrahydrides | VIPEr This in-class activity walks students through the preparation of a molecular-orbital diagram for methane in a square-planar environment. The students generate ligand-group orbitals (LGOs) for the set of 4 H(1s) orbitals and then interact these with carbon, ultimately finding that such a geometry is strongly disfavored because it does not maximize H/C bonding and leaves a lone pair on C.
10.3.5: Square-Planar Complexes - Chemistry LibreTexts Oct 25, 2022 ... That factor leads to square planar complexes generally adopting a low-spin configuration, which in this case means the lower orbitals are all ...
CHEM2P32 Lecture 11. Square and Tetrahedral Complexes - Brock University The orbital splitting diagram for square planar coordination can thus be derived from the octahedral diagram. As ligands move away along the z-axis, d-orbitals with a z-component will fall in energy. The d z2 orbital falls the most, as its electrons are concentrated in lobes along the z-axis.




![inorganic chemistry - Why is [PdCl4]2- square planar whereas ...](https://i.stack.imgur.com/xHv3g.png)







![d-orbital energy levels in planar [M II F 4 ] 2− , [M II (NH ...](https://pubs.rsc.org/image/article/2020/DT/d0dt02022b/d0dt02022b-f1_hi-res.gif)





0 Response to "45 square planar orbital diagram"
Post a Comment