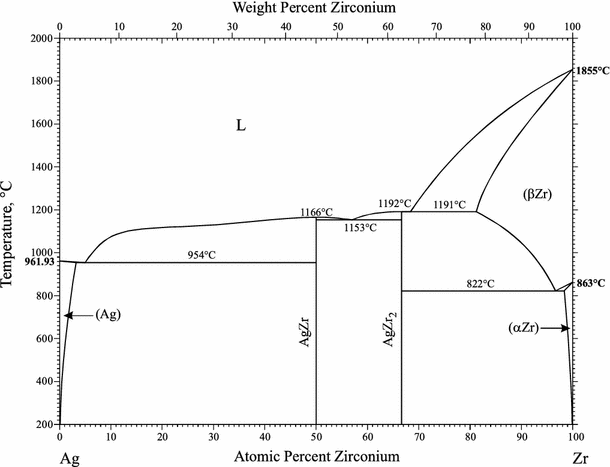
44 copper-silver phase diagram
Silver-copper phase diagram. | Download Scientific Diagram - ResearchGate The phase Fig. 1; the solubility limit is approximately 1.5 at % at a temperature of 570°С. After the melting process, an ingot was deformed by 50% during rolling. Each of the samples had a size... Metalloid - Wikipedia A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals.There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature of chemistry.
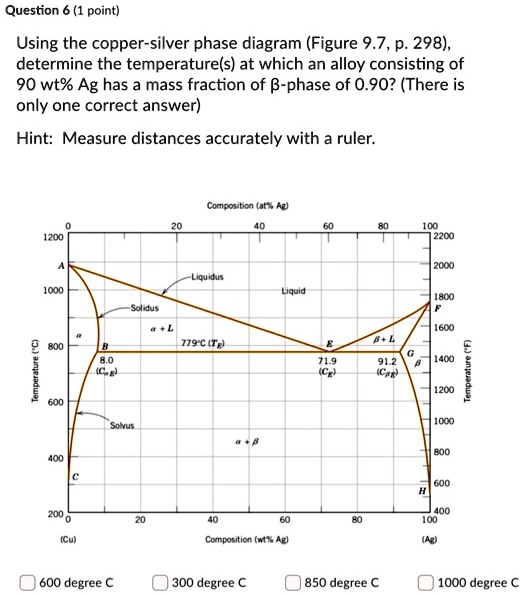
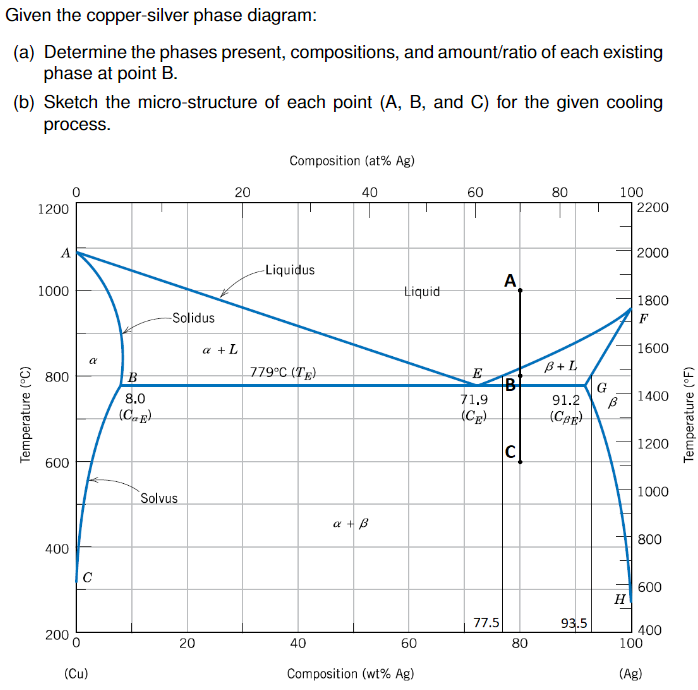
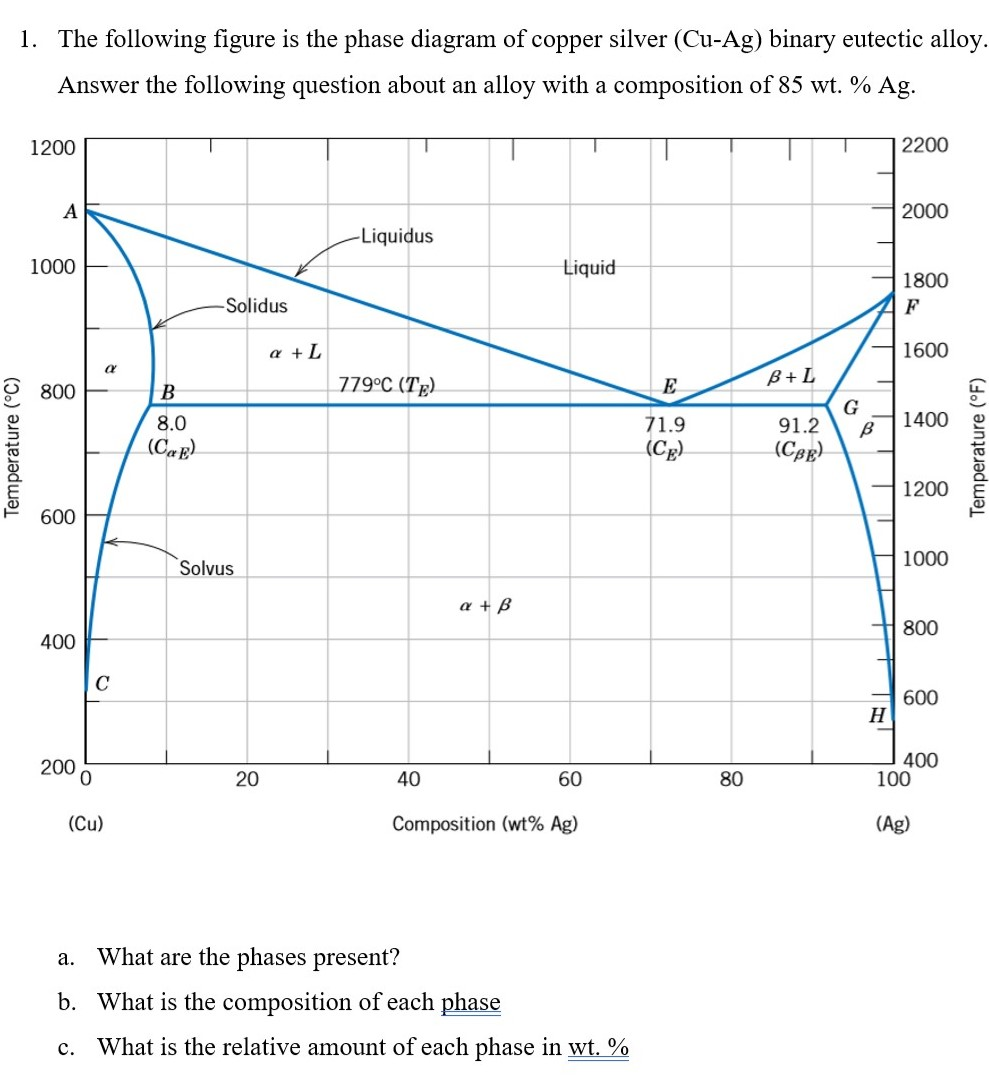
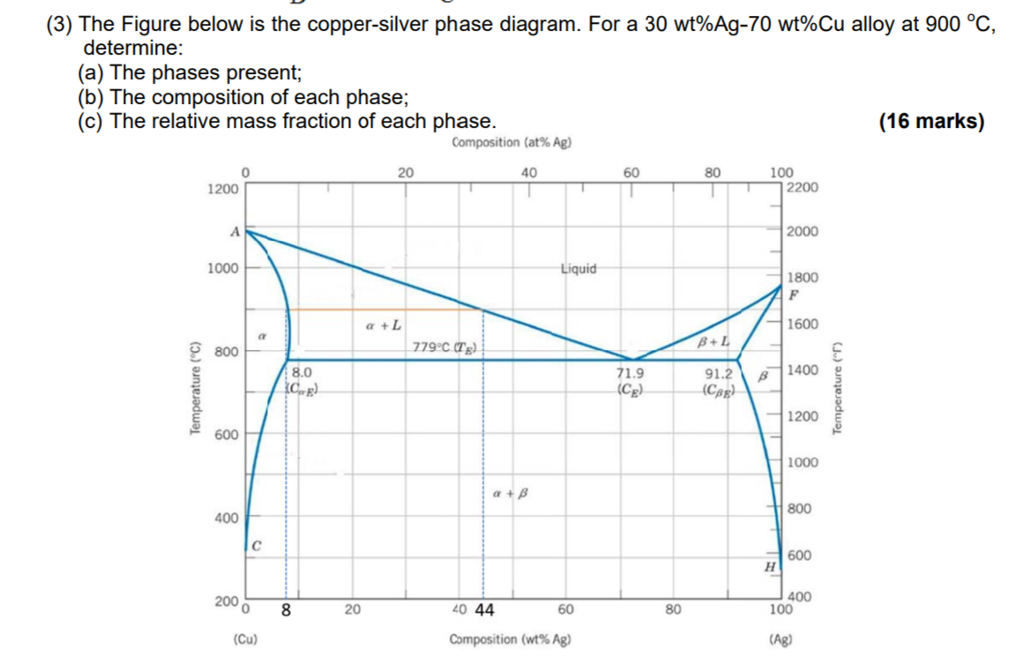
Solved 3. Consider the binary copper-silver phase diagram - Chegg (a) The phases present. (5 points) (b) The chemical composition of the phases. (5 points) (c) The mass fractions of each phase. (5 points) Show your work on the phase diagram. 2000 -Liquidus 1000 Liquid 1800 Solidus a + L 1600 ar 800 Question: 3. Consider the binary copper-silver phase diagram shown below.

Copper-silver phase diagram
Copper phase diagram - Big Chemical Encyclopedia Copper phase diagram The lead—copper phase diagram (1) is shown in Figure 9. Copper is an alloying element as well as an impurity in lead. The lead—copper system has a eutectic point at 0.06% copper and 326°C. In lead refining, the copper content can thus be reduced to about 0.08% merely by cooling. Further refining requites chemical treatment. Transforming heat transfer with thermal metamaterials and … Mar 12, 2021 · Phase-change materials can also be used for this purpose 154,155,156. In a thermal transistor (Fig. 4j ), a temperature bias on the gate is used to modulate the heat flux between the source and ... Phase Diagram - Industrial Metallurgists The phase diagram indicates that an iron-carbon alloy with 0.5% carbon held at 900 °C will consist of austenite, and that the same alloy held at 650 °C will consist of ferrite and cementite. Furthermore, the diagram indicates that as an alloy with 0.78% carbon is slow cooled from 900 °C, it will transform to ferrite and cementite at about 727 °C.
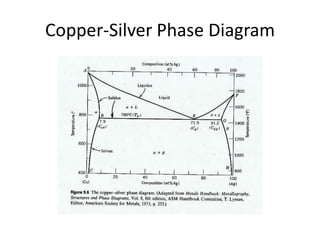
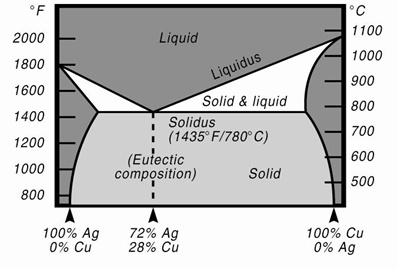
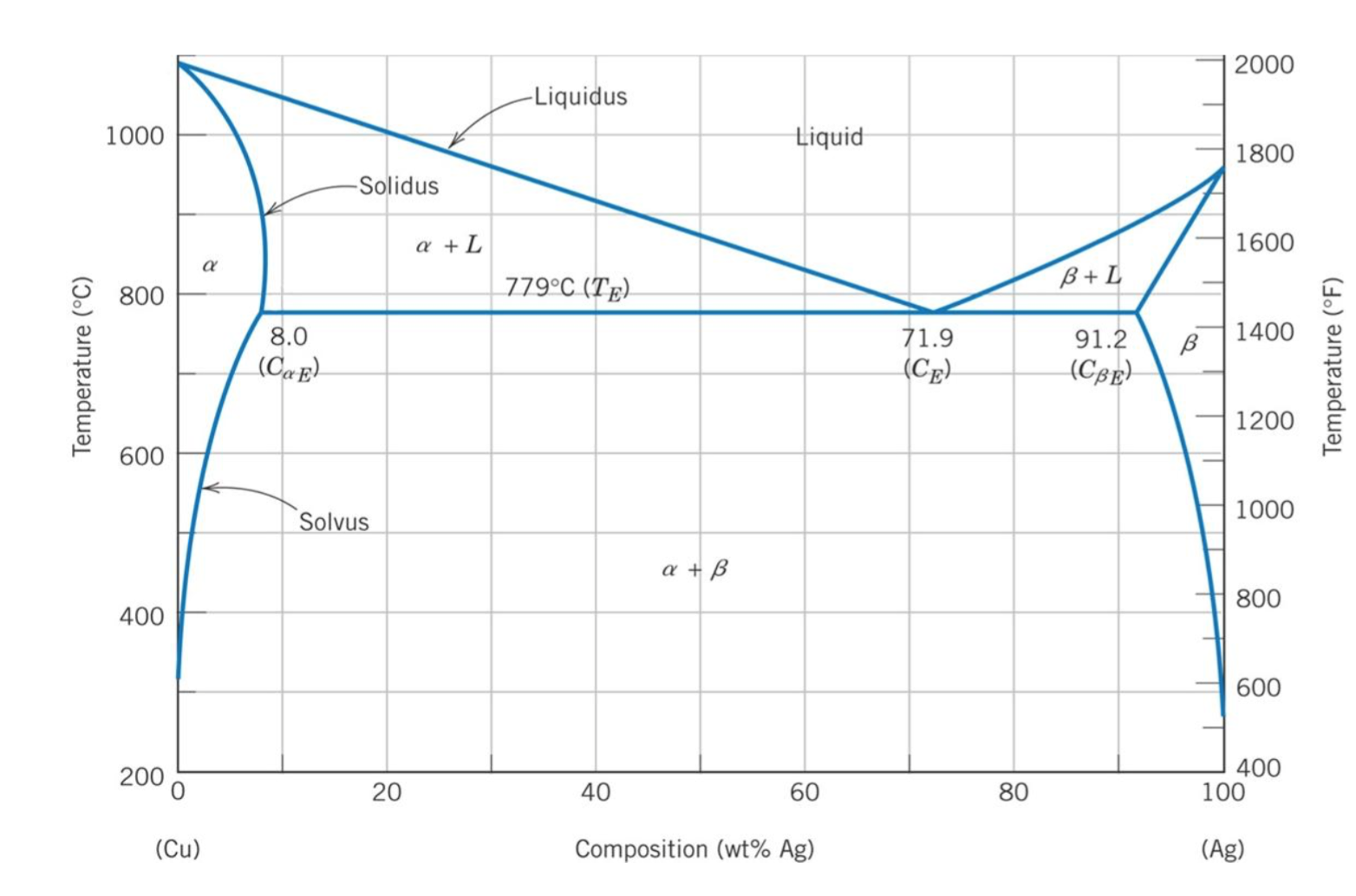
Copper-silver phase diagram. How To Read A Phase Diagram | Altair USA Phase diagrams are a great tool for the Brazing Engineer; the binary phase diagram is an invaluable tool for why a particular braze alloy and substrate interact the way they do. ... This is a fairly common characteristic of bimetallic alloys, the copper-silver system for instance has a phase diagram very similar to the one in the link. First ... Free PMP Practice Exam Questions - 120 Sample Test Questions Business Environment, 4.7 Close Project or Phase. A three-phase project to build a prototype is underway. The first phase entails design. In the second phase, the manufacturing team would produce the parts from the design specifications. In the third phase, the production team would assemble the parts to construct the prototype. The project is ... phase diagrams - SlideShare • Phase diagrams provide valuable information about melting, casting, crystallization and other phenomena. ... Copper-Silver Phase Diagram 26. • Solvus - (solid solubility line) BC, GH • Solidus - AB, FG, BEG (eutectic isotherm) • Liquidus - AEF • Maximum solubility: α = 8.0 wt% Ag, β = 8.8 wt %Cu • Invariant point (where 3 ... Standards & Properties: Metallurgy of Copper-Base Alloys Solid copper can be described as the arrangement of copper atoms in a face-centered-cubic (fcc) configuration. A copper atom is found at each corner and in the center of each face of a cube as depicted in Figure 1. This is the unit cell which is repeated in three dimensional space to make up the crystal structure of the metal.
Unit Cell & Lattice Parameter of Crystal Structure - Study.com May 13, 2022 · Copper, silver, gold, and the noble gases at sufficiently low temperatures are a few elements included in over twenty that form a face-centered cubic lattice structure. Metallurgy Matters: Phases, structures, and the ... - The Fabricator Figure 1 shows a typical silver-copper phase diagram and it tells you a number of things. First, at all temperatures above the liquid line, any combination of silver and copper is liquid. It also identifies where the solid of any combination of silver and copper exits as one or two phases. Improved CO2 reduction activity towards C2+ alcohols on a ... Oct 11, 2018 · The gas-phase products were analysed from the ... T. T. H. et al. Nanoporous copper–silver alloys by additive-controlled electrodeposition ... phase diagram and segregation. Nano Lett. 14, 6718 ... Engineering Electromagnetics - William Hayt.pdf - Academia.edu Physical Constants Quantity Value Electron charge e = (1.602 177 33 ± 0.000 000 46) × 10 −19 C Electron mass m = (9.109 389 7 ± 0.000 005 4) × 10 −31 kg Permittivity of free space 0 = 8.854 187 817 × 10 −12 F/m Permeability of free space µ 0 = 4π10 −7 H/m Velocity of light c = 2.997 924 58 × 10 8 m/s Dielectric Constant ( r ) and Loss Tangent ( / ) Material r /
[Solved] The copper-silver phase diagram is shown | SolutionInn The copper-silver phase diagram is shown in Figure 11-30. Copper has a higher melting point than silver. (a) Is copper element A or element B as labeled in the phase diagram? (b) Schematically draw the phase diagram and label all phases present in each region (single phase and two phase) of the phase diagram by writing directly on your sketch ... Silver-copper alloys, phase diagram - Big Chemical Encyclopedia The solid phases are pure crystals, as in Fig. 13.1. The right-hand diagram is for the silver-copper system and involves solid phases that are solid solutions (substimtional alloys of variable composition). The area labeled s is a solid solution that is mostly silver, and s is a solid solution that is mostly copper. [Solved] The copper-silver phase diagram is shown | SolutionInn The copper-silver phase diagram is shown in Figure 11-30. Copper has a higher melting point than silver. Refer to the silver-rich solid phase as gamma (γ) and the copper-rich solid phase as delta (δ). Denote the liquid as L.Figure 11-30 A phase diagram for elements A and B (for Problem 11-17). (a) For an overall composition of 60% B (40% A) The copper-silver phase diagram is shown in Figure 11-35. Copper has a ... The copper-silver phase diagram is shown in Figure 11-35. Copper has a higher melting point than silver. (a) Is copper element A or element B as labeled in the phase diagram? (b) Schematically draw the phase diagram and label all phases present in each region (single phase and two phase) of the phase diagram by writing directly on your sketch.
phasediagram - SlideShare Copper-Silver Phase Diagram 24. Peritectic Diagram • It is a phase diagram containing peritectic reaction. • peritectic reaction : L + α (s) = β (s) • In peritectic reaction, a liquid (L) and a solid (α) transform in to another solid (β) on cooling. 25. Phase diagram for Fe-C system (dotted lines represent iron-graphite equilibrium
13.2: Phase Diagrams- Binary Systems - Chemistry LibreTexts The left-hand diagram is for the binary system of chloroform and carbon tetrachloride, two liquids that form nearly ideal mixtures. The solid phases are pure crystals, as in Fig. 13.1. The right-hand diagram is for the silver-copper system and involves solid phases that are solid solutions (substitutional alloys of variable composition).
Could Call of Duty doom the Activision Blizzard deal? - Protocol Oct 14, 2022 · On Wednesday, the U.K.’s Competition and Markets Authority, one of three pivotal regulatory bodies arguably in a position to sink the acquisition, published a 76-page report detailing its review findings and justifying its decision last month to move its investigation into a more in-depth second phase.
Cu (Copper) Binary Alloy Phase Diagrams - ASM Digital Library Abstract This article is a compilation of binary alloy phase diagrams for which copper (Cu) is the first named element in the binary pair. The diagrams are presented with element compositions in weight percent. The atomic percent compositions are given in a secondary scale.
Copper-nickel Phase Diagram Pdf Three different phase regions (or fields) appear in the diagram: anα field, a liquid (L) field, and. Copper Aluminum Alloys This Al-Cu phase diagram shown only goes up to ca 60%, by weight, of Copper. and is "split" at around 54wt%Cu by a particular phase. This "split" means that the two parts of the diagram must be considered separately.
Order Panel - collepals.com - College Pal × Are you sure? NO YES YES
Question 29 use the copper silver phase diagram below Question 31 Use the copper-silver phase diagram below to answer the questions. In an experiment, one selected two samples of copper-silver alloy. One sample has 40 wt% ofsilver and 60wt% of copper and the other has 71.9 wt% silver and 28.1wt% copper. He performed the following processes: 1).
Solved Use the copper-silver phase diagram below to answer - Chegg Structures and Phase Diagrams. Vol. 8, 8th edition, ASM Handbook Committee, T. Lyman, Editor, American Society for Metals, 1973, p. 253.) In an experiment, one selected two samples of copper-silver alloy. One sample has 40 wt% of silver and 60wt% of copper and the other has 71.9 wt% silver and 28.1 wt% copper.
PART 4: COPPER SILVER SYSTEM PHASE DIAGRAM - YouTube In this lecture I have discussed phase diagram of copper silver system.
Interatomic Potentials Repository - NIST In particular, the entire Cu–Ag phase diagram calculated with the new potentials in conjunction with Monte Carlo simulations is in satisfactory agreement with experiment. This agreement suggests that EAM potentials accurately fit to 0 K properties can be capable of correctly predicting simple phase diagrams. Possible applications of the new potential set are outlined.
Components and phases | Micro structure | Binary eutectic systems ... Definitions and basic concepts Phases and microstructure Binary isomorphous systems (complete solid solubility) Binary eutectic systems Binary systems wit...
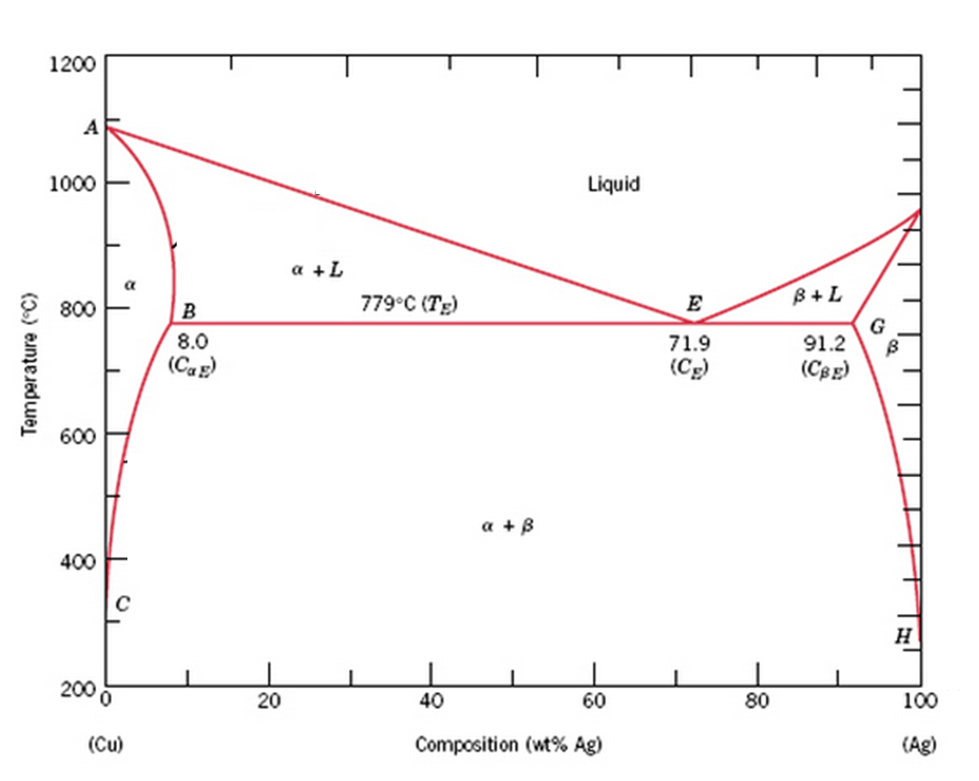
Figure 9. Cu-Ag Phase Diagram. The eutectic composition is 28.1 wt%... Cu-Ag Phase Diagram. The eutectic composition is 28.1 wt% Cu-71.9 wt% Ag and the solid solubility limit of silver in copper is 8 wt% Ag (after Hansen and Anderko 1958: 18) Source publication...
Phase Diagram: Meaning and Types | Material Engineering This phase diagram consists of two points, two lines and three areas. The two points of the two pure metals A & B. The upper line, obtained by connecting the points showing the beginning of solidification is called liquidius line, and the lower line, determined by connecting the points showing the end of solidification is called the solidus line.
(PDF) Coulson Richardson's Chemical Engineering Vol.6 … Coulson Richardson's Chemical Engineering Vol.6 Chemical Engineering Design 4th Edition
Phase Diagram | Explanation, Definition, Summary & Facts A phase diagram is a graphical representation of the substance phases, consists of the curved lines and the space between the two lines represent a specific phase of the matter at given pressure and temperature, whereas any point at the curve lines shows the equilibrium between two phases. Phase diagram explanation
Copper-Nickel Alloys: Properties, Processing, Applications 1.2 The Cu-Ni equilibrium diagram; ... A two-phase area in which liquid and a crystals co-exist is formed between liquidus and solidus. Below a dotted straight line at the bottom right, behaviour is ferromagnetic, above it is paramagnetic. ... tin-silver and tin-copper solders - e.g. S-Sn97Ag3 and S-Sn97Cu3 to EN 29453 - should be considered ...
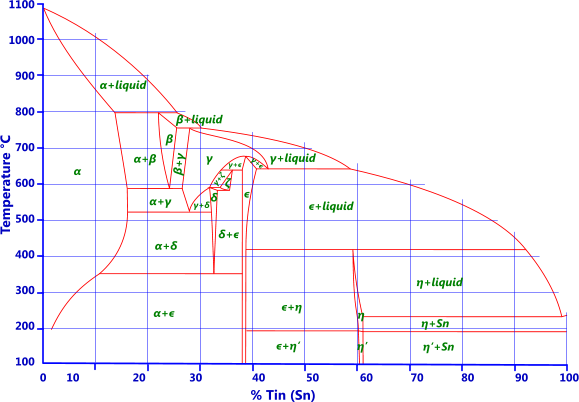
Some Phase Diagrams - Technische Fakultät Here are two phase diagrams for illustration purposes. I've chosen the diagrams for two old acquaintances: The system copper (Cu) - tin (Sn) The system copper (Cu) - zinc (Zn) Let's start with copper (Cu) - tin (Sn). This system contains what we call " bronze ". Here is the phase diagram: Phase diagram of copper (Cu) and tin (Sn)
What's New in Phase Diagram - FactSage C phase diagram of the Fe-Cr-C system at 2.8 Wt.% Cr calculated under normal equilibrium conditions (orthoequilibrium). Data are taken from the SGTE 2011 databases . The second figure is the paraequilibrium diagram for the same system when C is the only diffusing component.
RS-25 - Wikipedia The RS-25 engine consists of pumps, valves, and other components working in concert to produce thrust. Fuel (liquid hydrogen) and oxidizer (liquid oxygen) from the Space Shuttle's external tank entered the orbiter at the umbilical disconnect valves and from there flowed through the orbiter's main propulsion system (MPS) feed lines; whereas in the Space Launch System (SLS), fuel and oxidizer ...
The copper-silver phase diagram is shown in Figure 11-30. The copper-silver phase diagram is shown in Figure 11-30. Copper has a higher melting point than silver. (a) Is copper element A or element B as labeled in the phase diagram? (b) Schematically draw the phase diagram and label all phases present in each region (single phase and two phase) of the phase diagram by writing directly on your sketch ...
Question 20 use the copper silver phase diagram below - Course Hero Question 20 Use the copper-silver phase diagram below to answer the questions. 2.5 out of 2.5 points Selected Answer: A. A. B. C. D. Liquid. Liquid. α-solid + liquid. α-solid +α'solid +β-solid. α-solid + β-solid.Answers:In an experiment, one selected two samples of copper-silver alloy.
Phase Diagram - Industrial Metallurgists The phase diagram indicates that an iron-carbon alloy with 0.5% carbon held at 900 °C will consist of austenite, and that the same alloy held at 650 °C will consist of ferrite and cementite. Furthermore, the diagram indicates that as an alloy with 0.78% carbon is slow cooled from 900 °C, it will transform to ferrite and cementite at about 727 °C.
Transforming heat transfer with thermal metamaterials and … Mar 12, 2021 · Phase-change materials can also be used for this purpose 154,155,156. In a thermal transistor (Fig. 4j ), a temperature bias on the gate is used to modulate the heat flux between the source and ...
Copper phase diagram - Big Chemical Encyclopedia Copper phase diagram The lead—copper phase diagram (1) is shown in Figure 9. Copper is an alloying element as well as an impurity in lead. The lead—copper system has a eutectic point at 0.06% copper and 326°C. In lead refining, the copper content can thus be reduced to about 0.08% merely by cooling. Further refining requites chemical treatment.












![The copper-silver alloy binary phase diagram [17]. | Download ...](https://www.researchgate.net/publication/345830319/figure/fig2/AS:957647859773440@1605332450279/The-copper-silver-alloy-binary-phase-diagram-17.png)





0 Response to "44 copper-silver phase diagram"
Post a Comment