44 potential energy diagram catalyst
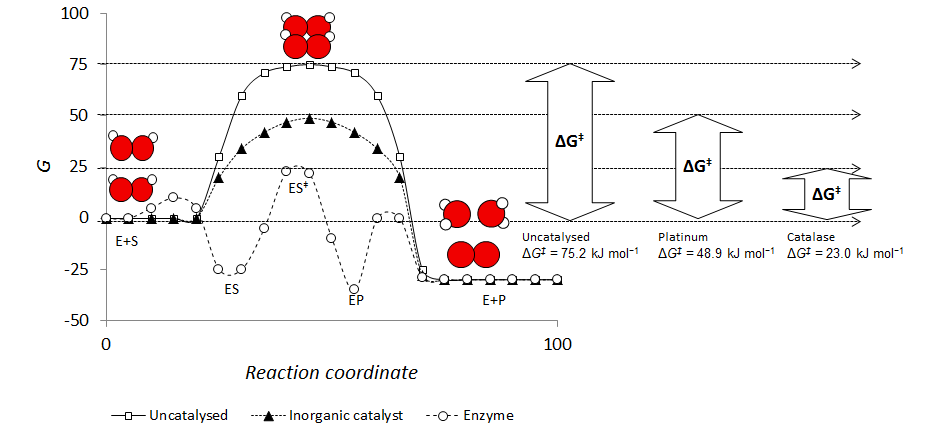
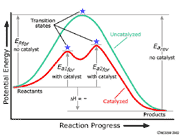
Explain with the help of a potential energy diagram that ... The potential energy diagram compares the potential energy barriers for the catalysed and uncatalysed reactions. The barrier for uncatalysed reaction (E a) is larger than that for the same reaction in the presence of a catalyst E a. › eere › fuelcellsFuel Cells | Department of Energy Fuel cells have several benefits over conventional combustion-based technologies currently used in many power plants and vehicles. Fuel cells can operate at higher efficiencies than combustion engines and can convert the chemical energy in the fuel directly to electrical energy with efficiencies capable of exceeding 60%.
Potential Energy Diagrams Flashcards - Quizlet potential energy diagram. One change in a reaction, other than adding a catalyst, that can increase the rate of the given reaction. is a change in temperature. One of the basic concepts of kinetics is that in order for a reaction to occur. reactant particles must collide.
Potential energy diagram catalyst
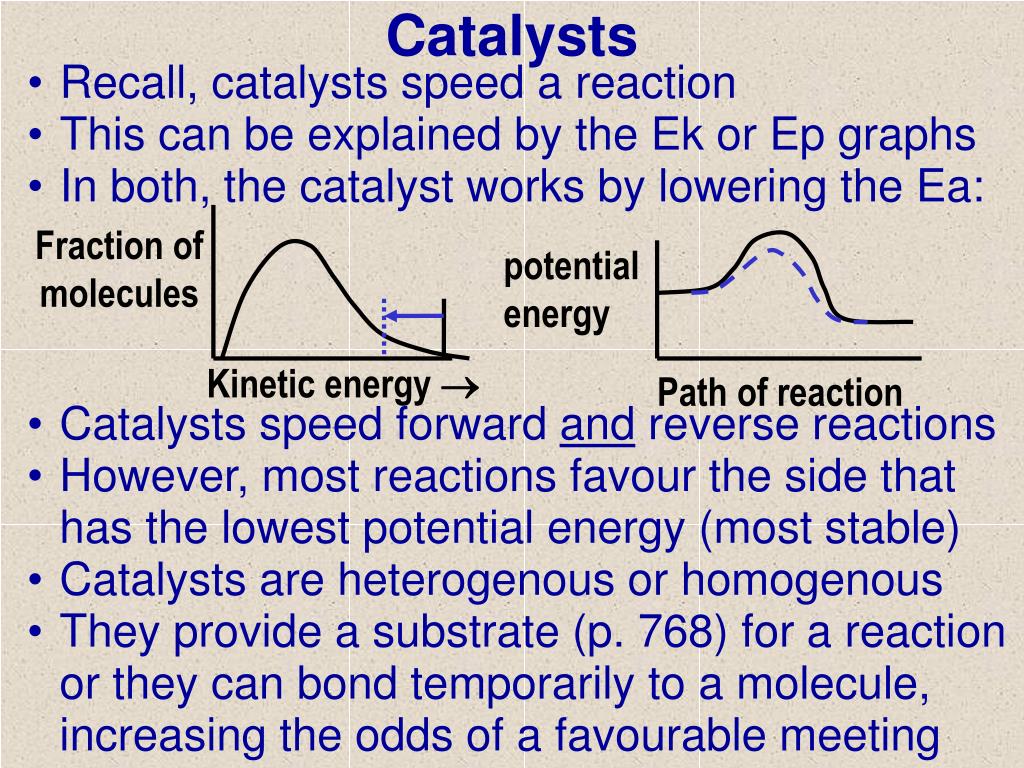
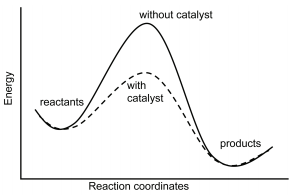
Dublin Schools - Lesson : Catalysts Potential Energy Diagram The graph shows a reaction rate with and without the use of a catalyst. Summary A catalyst is a substance that increases the rate of a chemical reaction. A catalyst provides an alternate pathway for the reaction that has a lower activation energy. PDF Kinetics Quiz 4 Potential Energy Diagrams B. Decreasing ... Kinetics Quiz 4 Potential Energy Diagrams 1. A catalyst increases the rate of a reaction by A. Increasing the concentration of the reactant(s) B. Decreasing the concentration of the reactant(s) C. Increasing the activation energy of the overall reaction D. Decreasing the activation energy of the overall reaction 2. 12.7 Catalysis - Chemistry 2e - OpenStax Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams List examples of catalysis in natural and industrial processes Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst , a substance that can increase the reaction rate without being ...
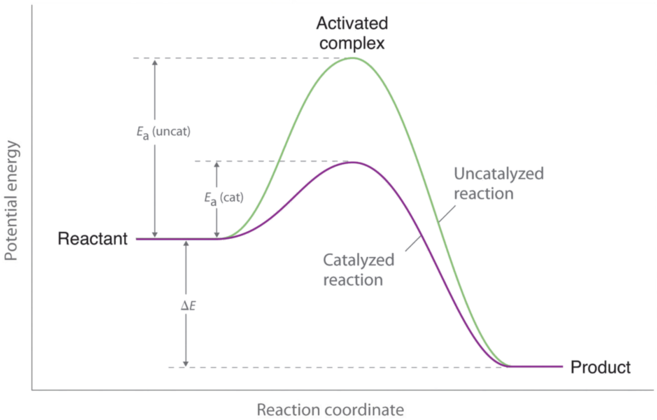
Potential energy diagram catalyst. Physics notes for class 11 - Physicscatalyst Master Class 11 Physics And Be Successful in exams. Here find Notes, assignments, concept maps and lots of study material for easy learning and understanding. 12.7 Catalysis - Chemistry - opentextbc.ca This indicates the use of a catalyst in diagram (b). The activation energy is the difference between the energy of the starting reagents and the transition state—a maximum on the reaction coordinate diagram. The reagents are at 6 kJ and the transition state is at 20 kJ, so the activation energy can be calculated as follows: catalysis - Potential Energy Diagrams and the Effect of ... Why does a potential energy diagram showing the effect of a catalyst on activation energy not move left on the reaction pathway scale (compared to uncatalysed reaction) if a catalyst speeds up reac... PDF Potential Energy Diagrams POTENTIAL ENERGY DIAGRAMS PURPOSE POTENTIAL ENERGY DIAGRAMS ARE A VISUAL REPRESENTATION OF POTENTIAL ENERGY IN A CHEMICAL REACTION NOTE THE X AXIS IS USUALLY REACTION CORDINATE OR TIME NOTE THE Y AXIS IS POTENTIAL ENERGY WHERE IN THE REACTION PROGRESS/TIME IS THE POTENTIAL ENERGY HIGHEST? PURPOSE CONT.
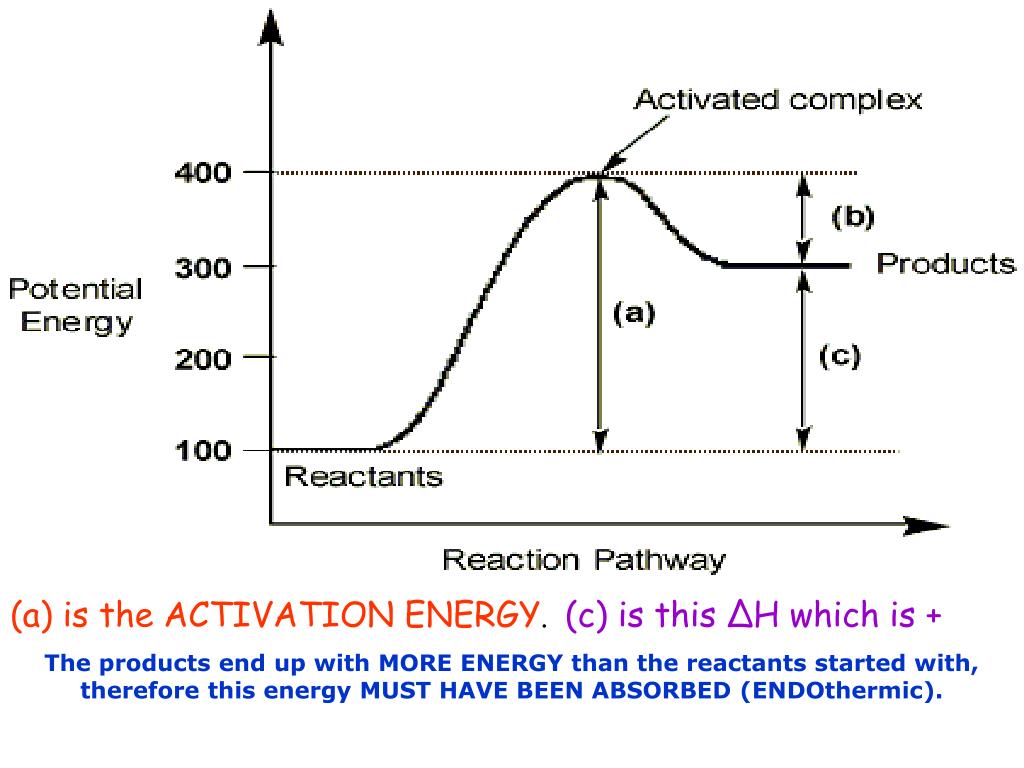
Potential Energy Diagrams | Chemistry for Non-Majors Figure 1. A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. Given the potential energy diagram for a reaction: Which ... 01/10/2017 Chemistry High School answered • expert verified Given the potential energy diagram for a reaction: Which intervals are affected by the addition of a catalyst? (1) 1 and 2 (2) 1 and 3 (3) 2 and 4 (4) 3 and 4 2 See answers Advertisement priyankatutor Intervals 1 and 3 are most affected by the addition of catalyst. Option 2 is correct. 1 Potential energy diagram of a heterogeneous catalytic ... Download scientific diagram | 1 Potential energy diagram of a heterogeneous catalytic reaction (A + B → P) with gaseous reactants (A, B), product (P), and solid metal catalyst from publication ... kentchemistry.com › links › KineticsPotential Energy Diagrams - Kentchemistry.com A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.
Financial Opportunities: Funding Opportunity Exchange - Energy Geothermal resources can be found nationwide, are “always on,” and represent vast domestic energy potential. Only a fraction of this potential has been realized due to technical and non-technical barriers that constrain industry growth. The U.S. Department of Energy’s Geothermal Technologies Office’s GeoVision report concluded that with technology improvements, … Potential Energy Diagrams Flashcards | Quizlet Endothermic PE Diagram. a chemical reaction where the Potential Energy of the product (s) is higher than that of the reactant (s). The chemical equation is going to represent energy wirtten with the reactant (s) or substracted from the product (s) Catalyst. Substance that decreases activation energy and increases reaction rate in a chemical ... Solved What is the catalyst? Sketch a potential energy ... Sketch a potential energy diagram and use it to explain how addition of the catalyst affects a hypothetical chemical reaction A + B --> C + D. This problem has been solved! See the answer See the answer See the answer done loading How would a catalyst affect the potential energy diagram ... Adding a Catalyst to a reaction decreases the energy required be the reactants to react. How do you draw a Potential Energy Diagram? To draw a potential energy diagram, one must plot a graph....
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
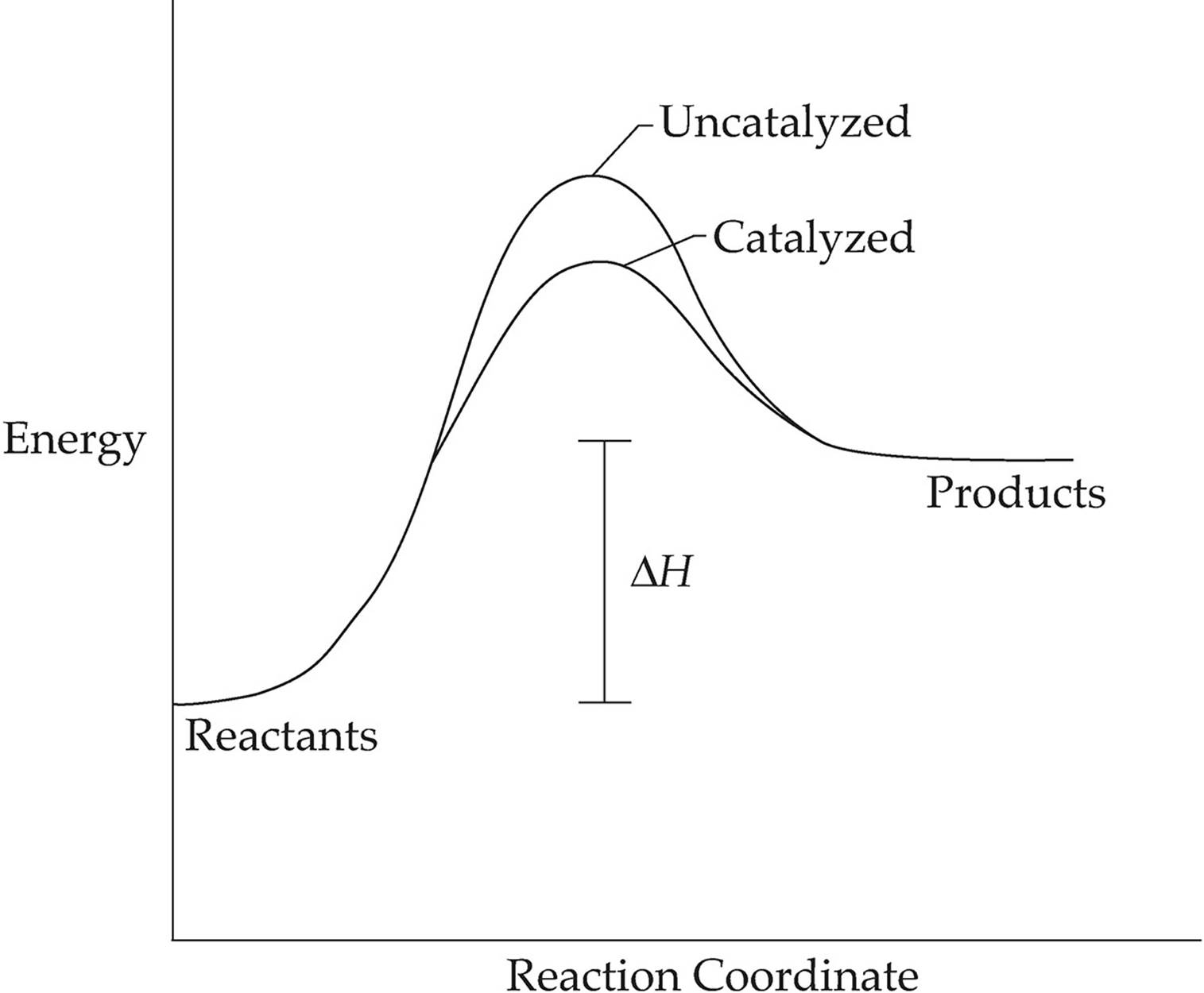
What is the potential energy diagram for catalyzed and ... Answer: The overall diagram will depend on whether the reaction is endothermic (final H is higher then initial H) or exothermic (final H is lower than initial H) BUT: Catalysed reactions lower the activation energy - the hump that needs to be overcome for the reaction to proceed. (Catalysts ofte...
PDF Chemical Kinetics - GUSD Examine the following Potential Energy Diagrams then answer the questions that follow. c d + e 1. Which of the reactions has the greatest H (or heat of reaction)? _____ 2. Which reaction has the greatest Activation Energy? _____ 3. For which reactions might we want to use a catalyst to increase the rate of reaction? _____ 4.
PDF Chemical kinetics Name: Date - The Leon M. Goldstein High ... In the potential energy diagram, which letter represents the potential energy of the activated complex? A. A B. B C. C D. D 2. The addition of a catalyst to a reaction will cause a change in the A. potential energy of the reactants B. potential energy of the products C. heat of reaction D. activation energy 3.
PDF Name: Off. Class: Per: Date: Teacher: PE Diagram Regents ... the balanced equation below represents the reaction between carbon monoxide and oxygen to produce carbon dioxide. 2co(g) + o2(g) 2co2+ energy 7.on the potential energy diagram below, draw a dashed line to show how the potential energy diagram changes when the reaction is catalyzed. 8.on the potential energy diagram below, draw a double-headed …
Answer the following in brief. How a catalyst increases ... Potential energy barriers for catalyzed and uncatalyzed reactions. ii. The potential energy diagram compares the potential energy barriers for the catalysed and uncatalysed reactions. The barrier for uncatalysed reaction (E a) is larger than that for the same reaction in the presence of a catalyst E a. iii.
PDF The Leon M. Goldstein High School for the Sciences Potential Energy Diagram pathway without with catalyst Course Ca culate the heat o reaction. What effect does a catalyst have in a potential energy diagram? Does the. potential energy of-the products or reactants change with the addition of a catalyst? Find the activation energy for the catalyzed and un-catalyzed reaction.
Potential Energy Diagram of Catalyzed and Uncatalyzed ... Analyzing the potential energy diagram of a regular/uncatalyzed and a catalyzed (adding a catalyst) reaction. Remember that the 🔼H of reaction remains the s...
12.7 Catalysis | Chemistry - Lumen Learning This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy.
Which of the following phase diagrams represents how a ... A regular exothermic potential energy diagram is shown, with a dotted line representing how the potential energy diagram changes with the use of a catalyst. The dotted line shows a new potential energy diagram with two activation energy hills, the second taller than the first, instead of the one hill in the regular potential energy diagram.
Solved Given the potential energy diagram below, calculate ... Without catalyst 180 160 140 120 With catalyst Potential Energy (kJ) 100 80 60 40 20 0 Reaction coordinate Type your numeric answer and submit Given the potential energy diagram, calculate the activation energy for the uncatalyzed reaction (in kJ) Without catalyst This problem has been solved! See the answer Show transcribed image text
Catalysis - Chemistry Consequently, the presence of a catalyst will permit a system to reach equilibrium more quickly, but it has no effect on the position of the equilibrium as reflected in the value of its equilibrium constant (see the later chapter on chemical equilibrium). This potential energy diagram shows the effect of a catalyst on the activation energy.
PDF Potential Energy Diagram Worksheet ANSWERS Potential Energy Diagram Worksheet ANSWERS 1. Which of the letters a-f in the diagram represents the potential ... A catalyst changes the reaction mechanism, in the process lowering the activation energy. 5. Name 4 things that will speed up or slow down a chemical reaction.
Potential energy diagram with/without catalyst in a ... Potential energy diagram with/without catalyst in a hypothetical exothermic chemical reaction coordinate of Boltzmann distribution. The presence of the catalyst opens a different reaction pathway...
😊 Explain the effect of ph on enzyme activity. How does pH affect the rate of enzyme activity ...
12.7 Catalysis - Chemistry 2e - OpenStax Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams List examples of catalysis in natural and industrial processes Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst , a substance that can increase the reaction rate without being ...
PDF Kinetics Quiz 4 Potential Energy Diagrams B. Decreasing ... Kinetics Quiz 4 Potential Energy Diagrams 1. A catalyst increases the rate of a reaction by A. Increasing the concentration of the reactant(s) B. Decreasing the concentration of the reactant(s) C. Increasing the activation energy of the overall reaction D. Decreasing the activation energy of the overall reaction 2.


0 Response to "44 potential energy diagram catalyst"
Post a Comment