43 co2 orbital overlap diagram
how to draw molecular orbital diagram of co - Earn A Lot ... Co2 Orbital Diagram. Draw the orbital diagram of Co. Electronic configuration of O atom. In this case were using the standard one. D the bond order is 3. First step is to determine which MO diagram were using. The 7 Rules of Drawing Molecular Orbitals. Molecular orbitals in Carbon Monoxide - ChemTube3D Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry ...
OF2 Lewis Structure, Molecular Geometry, Hybridization ... Now, we will discuss the steps to form the Lewis Structure of OF2. Steps to form OF2 Lewis Structure Diagram Step 1: Find the Total number of Valence Electrons. The first and foremost step is to calculate the total number of valence electrons in an OF2 molecule. Oxygen belongs to group 16, the chalcogen family, and has a valency of 6.

Co2 orbital overlap diagram
CO2 Lewis Structure (2021 UPDATED) All You Need To Know 6 Steps on How to Draw CO2's Lewis Structure Calculate the total valence electrons found in a molecule. Carbon Valence Electron=4 Oxygen Valence electrons: 6*2 = 12 Total number of valence electrons = 16 Find the central atom, which is usually the one with the highest bonding sites, is the Carbon atom. Draw four dots around it. COCl2 Lewis Structure, Molecular Geometry, Hybridization ... It gives us a graphical sketch with electron-dot notations for us to grasp the process in a simple manner. Step 1: The initial step is to calculate the valence or outermost shell electrons in a molecule of COCl2. If we look at the periodic table, we can see that C belongs to group 14 and has an atomic number of 6. Co2+ Orbital Diagram The angular overlap diagrams for the molecular orbitals with high d orbital .. For Co2+: High-spin octahedral d7 has LFSE = -∆o. Tetrahedral d7 has. As it is sometimes explained, the statement that 4 s orbital is lower in energy than 3 d But while you fill 3 d orbital with electrons it becomes lower and lower in. Part B.
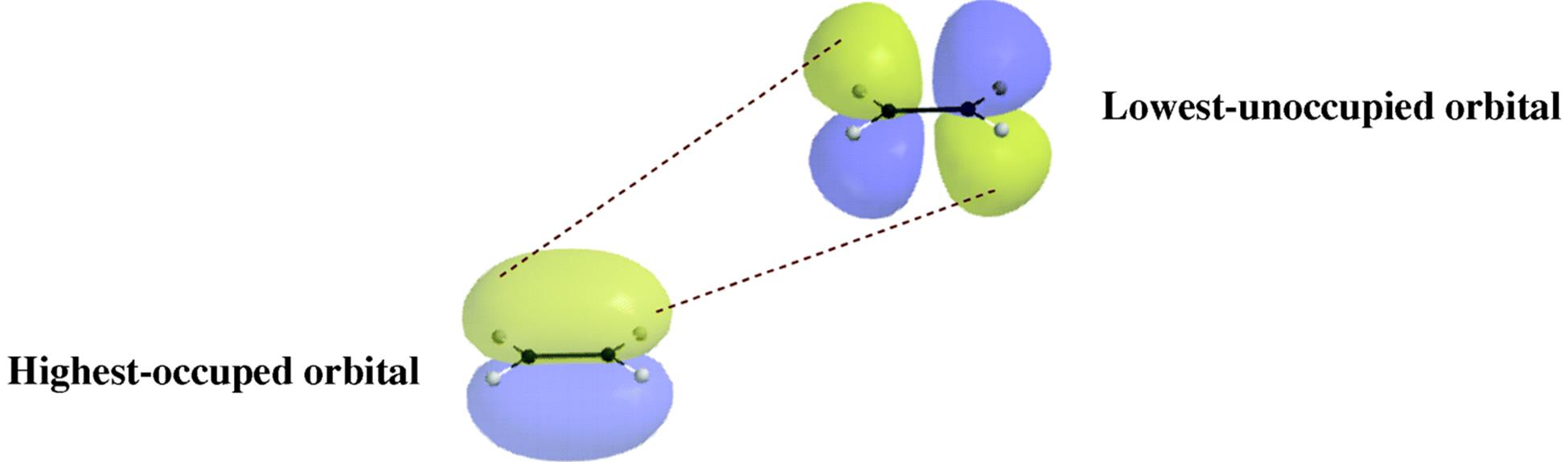
Co2 orbital overlap diagram. Molecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. Draw The Orbital Diagram For The Ion Co2+ - schematron.org Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click within an orbital to add electrons. Part C. Draw the orbital diagram for the ion N3−. What the orbital overlap diagram for SO2 NH3 is a molecule with a nitrogen atom in the middle surrounded by 3 hydrogen atoms bonded covalently. Nitrogen has 5 valence electrons so, it will form sigma bonds (sp3 hybrid orbitals) with each of the hydrogen atom. The remaining lone pair electrons will be situated above the atom. You ca look at this image I've pulled from Google.
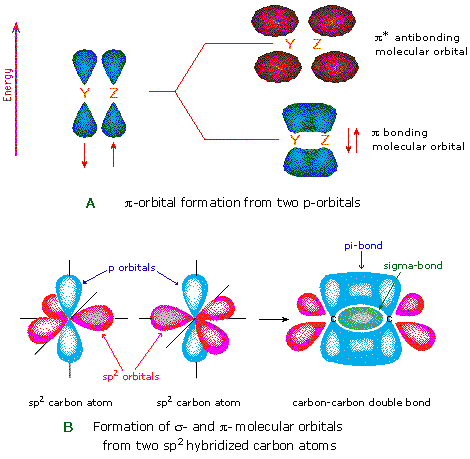
In, detals. How are the atomic orbitals overlapped in CO? Answer: The pi bonds in CO2 are the ones formed by lateral overlapping of the two p orbitals on C and the p orbital on each O atom. The sigma bonds are formed by the head on overlapping of the sp hybridized orbitals of C and one of the sp2 hybridized orbitals of each O atom. Since C is the centra... CO2 Lewis Structure, Molecular Geometry and Hybridization These two hybridized orbitals overlap with the two p-orbitals of the Oxygen atom that results in the formation of sigma bonds. Remaining electrons in the p-orbitals in the Oxygen atom form pi bonds. As sp orbitals are hybridized to form the bonds, CO2 has an sp hybridization. CO2 Molecular Geometry SCl6, ICl2-, ICl4+, CO2, C2H2 & C2H4 : ORBITAL OVERLAPPING ... Lets learn how to draw the orbital Overlapping Diagram of these molecules:00:01 SCl604:08 ICl2-07:27 ICl4+10:36 CO214:40 C2H221:29 C2H4 Hybridization of CO2 - Explanation, Properties, Types ... The formation of CO2 consists of two particles: Oxygen and Carbon. Carbon is in group 4, whereas oxygen is in group 6. Furthermore, there are 2 Oxygen atoms. Therefore, CO2= 4 + 6 (2) = 16. So, the total valence electrons are 16. Carbon is the least electronegative, which means it stays at the centre. So, place the Carbon in the middle and then ...
13.3. Molecular orbitals for three-carbon systems ... If we just take the π molecular orbital and not any of the s, we get three of them. π 1 is bonding with no nodes, π 2 is nonbonding (In other words, the same energy as a regular p-orbital) with a node, and π 3 is antibonding with 2 nodes (none of the orbitals are interacting). The first two electrons will go into the π 1 molecular orbital, regardless of whether it is a cation, radical, or ... When we draw an MO diagram of CO or CO2, why is the ... Answer (1 of 3): For a heteronuclear molecule with less than atomic no. 10. Z effective experiences by the electrons of two atoms are not the same. The electrons ... CO2 : ORBITAL OVERLAPPING DIAGRAM - YouTube Some corrections from previous video.. Sorry for the unintentional mistakes i made. So here is the correct one ok. Overlapping of orbitals and its types, s-s, p-p, and s-p ... Diagram : 1 S Orbital 1 S Orbital S-S overlap. p - p orbital overlap (Formation of Fluorine F 2 molecule): The mutual overlap between two half-filled p - orbitals of two atoms is called p - p overlap and the covalent bond formed is known as p - p bond. If the overlapping takes place along the internuclear axis the bond is called sigma ...
how to draw molecular orbital diagram for co2 - Corinne Haas Based on the amount of orbital overlap the relative changes in energy differ going from the atomic orbital to the molecular. Since more than one atom is involved we refer to these orbitals as molecular orbitals. Molecular Orbital Diagram Of Polyatomic Co2 Molecules Chemical Bonding Molecular Structures Youtube
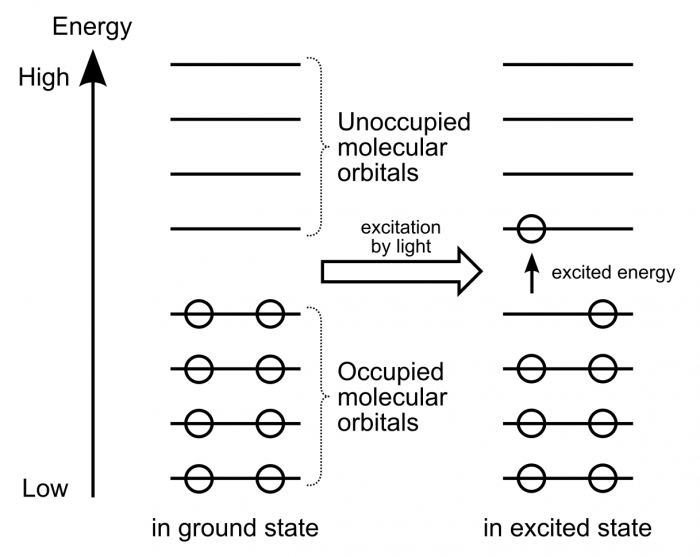
Carbon Monoxide Molecular Orbital Diagram Explanation A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not. . combinations such as CO and NO show that the 3σg MO is higher in energy. Mulliken came up with theory known as Molecular Orbital Theory to explain questions like above.
Orbital Overlap Concept - Overlapping of Atomic Orbitals ... When two atoms come in contact with each other to form a bond, their overlap can be positive, negative or even zero depending upon the phase and sign of the two interacting orbital. Positive Overlapping of Atomic Orbital - When the phase of two interacting orbitals is same, then the overlap is positive and in this case, the bond is formed.
37 draw the orbital diagram for the ion co2+ - Diagram ... CO2 Molecular Orbital (MO) Diagram. The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals. It also helps us to find the bond order, bond length, bond strength of the molecule. In the diagram, the left-hand side consists of the atomic orbitals of carbon.
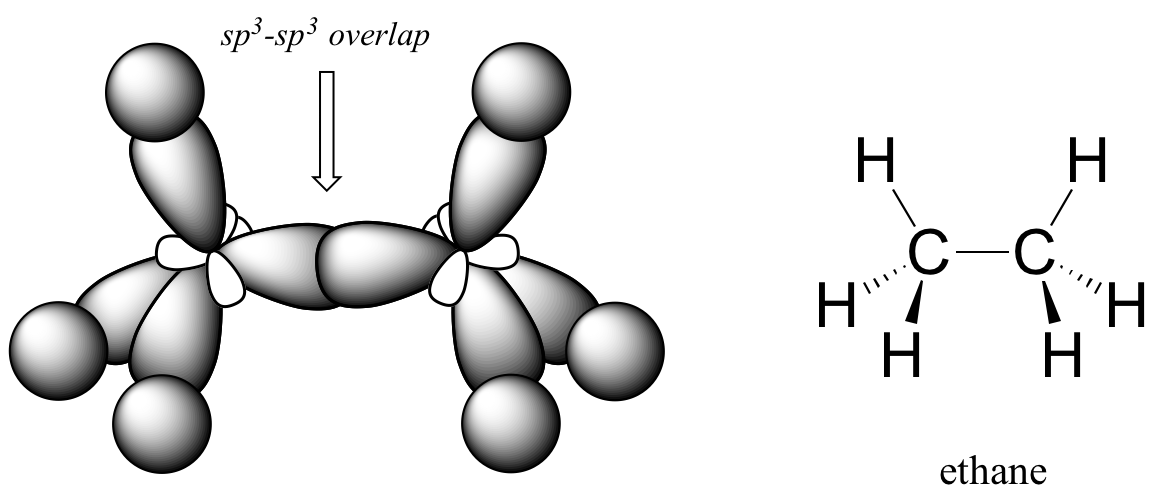
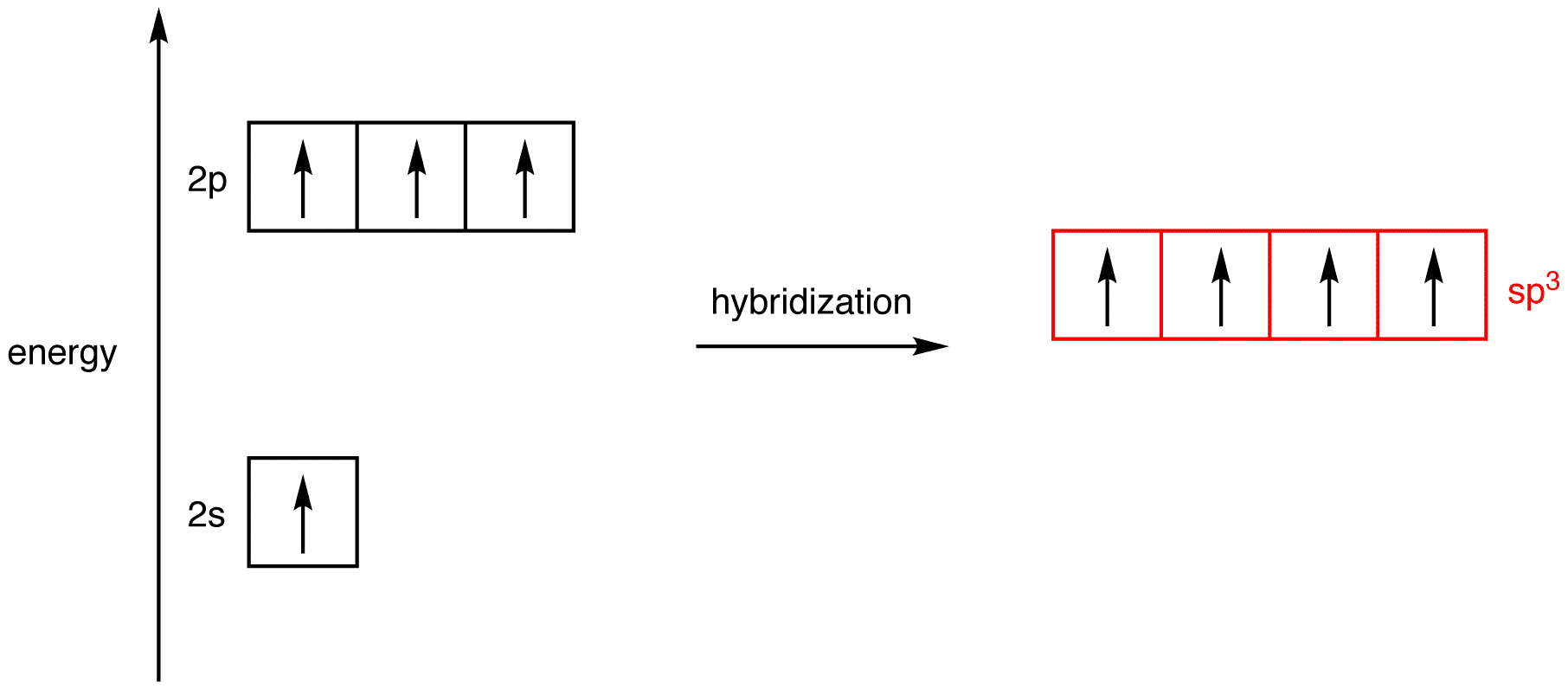
Hybrid Orbitals - Chemistry LibreTexts Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is tetrahedral, since that minimizes electron ...
How To Draw Molecular Orbital Diagram Of Co - Drawing ... Electronic configuration of co molecule is: Draw the orbital diagram for the ion co2+. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The bonding mos are the 2σ, 1πx, 1πy, and 3σ, which gives 2 +2 +2 +2 = 8 bonding electrons. The content is presented using short focussed and interactive screencast.
Molecular Orbitals for Carbon Monoxide - Newcastle University For the three occupied σ orbitals, for each pair of C—O NAO overlaps, their contribution to bonding c1c2S12 is shown in the Table of Relative Contributions of Overlaps to Bonding. S12 is the overlap integral between them calculated in the NBO analysis and c1 and c2 are their LCAO coefficients given in the first Table
PDF 2266 Walsh The Electronic Orbitals, Shapes, 2268 Walsh : The Electronic Orbitals, Shapes, nnd molecule. The first of these (b,") is built from a fi atomic orbital on each of the three atoms overlapping in-phase. It is At-tB and (weakly) Bt+B bonding, and becomes one of the lower xu orbitals in the linear molecule.The A atomic orbital concerned in it is pure fi in both bent and linear molecules.
Hybridization of CO2 - Hybridization of C, O in Carbon Dioxide Now, these sp hybridized orbitals of the carbon atom overlap with two p orbitals of the oxygen atoms to form 2 sigma bonds. As for the two remaining p electrons they will be used to form a pi bond. In carbon dioxide molecule, oxygen also hybridizes its orbitals to form three sp 2 hybrid orbitals.
What is linear combination of atomic orbitals for CO2 ... They are shown in its molecular orbital diagram. For example, consider the overlap of the carbon 2s with the 2pz group orbitals of 2 × O. That is labeled 3ag, and is given by Ψ 3ag = 1 √3 [ψ(2s)(C) + ψ(2pz)(O(1)) −ψ(2pz)(O(2))] where ψ is each atomic orbital wave function and Ψ is the molecular orbital wave function.
Co2+ Orbital Diagram The angular overlap diagrams for the molecular orbitals with high d orbital .. For Co2+: High-spin octahedral d7 has LFSE = -∆o. Tetrahedral d7 has. As it is sometimes explained, the statement that 4 s orbital is lower in energy than 3 d But while you fill 3 d orbital with electrons it becomes lower and lower in. Part B.
COCl2 Lewis Structure, Molecular Geometry, Hybridization ... It gives us a graphical sketch with electron-dot notations for us to grasp the process in a simple manner. Step 1: The initial step is to calculate the valence or outermost shell electrons in a molecule of COCl2. If we look at the periodic table, we can see that C belongs to group 14 and has an atomic number of 6.
CO2 Lewis Structure (2021 UPDATED) All You Need To Know 6 Steps on How to Draw CO2's Lewis Structure Calculate the total valence electrons found in a molecule. Carbon Valence Electron=4 Oxygen Valence electrons: 6*2 = 12 Total number of valence electrons = 16 Find the central atom, which is usually the one with the highest bonding sites, is the Carbon atom. Draw four dots around it.








0 Response to "43 co2 orbital overlap diagram"
Post a Comment