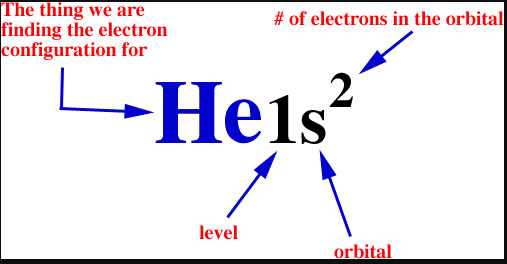
40 what does each box in an orbital diagram represent
Chap 6 part Flashcards - Quizlet What does each box in an orbital diagram represent? An orbital. What quantity is presented by the half arrows in an orbital diagram? Each arrow represents an electron while the direction of the arrow represents the electron spin. Aufbau Principle. Orbital Diagrams Chemistry Tutorial - AUS-e-TUTE An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
Build an Atom - Atoms | Atomic Structure | Isotope ... - PhET Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
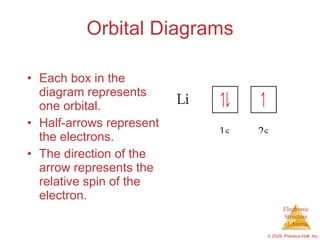
What does each box in an orbital diagram represent
CHEMISTRY CIRRITO CHAPTER 5 Flashcards - Quizlet What does each small box in the diagram represent? the orbital. How many electrons can each orbital hold? 2. How many electrons can the d sublevel hold? 10. Which is associated with more energy: the 2s or the 2p orbital? 2p. Which energy level has the least amount of energy? 1s. Learn About Box Diagram | Chegg.com Each box represents a single orbital and the number of spins in each box represents the number of electrons in that particular orbital. S-orbital can accommodate 2 electrons. P-orbital has three orientations and so can accommodate 6 electrons. D-orbital has 5 orientations and so can accommodate 10 electrons. (PDF) University Physics Volume1-OP | Rimit ... - Academia.edu Academia.edu is a platform for academics to share research papers.
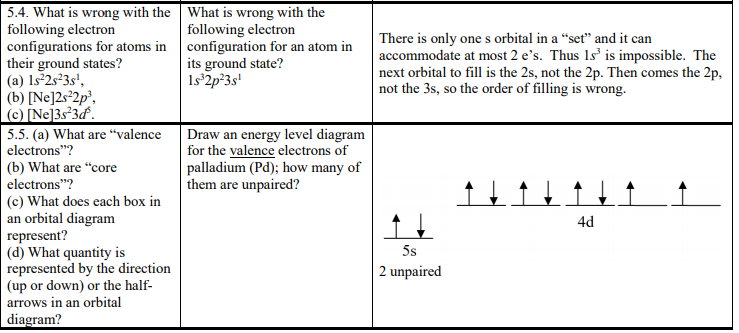
What does each box in an orbital diagram represent. Quantum Number Questions and Answers | Study.com The g-orbital block comes after the f-orbital block and consists of elements that have not been synthesized yet. Provide the full set of 3 quantum numbers (n, l, and ml) for all the possible g-bloc... OneClass: (a) What are "valence electrons"? (b) What are ... Write the complete orbital diagram for each of the following elements, using boxes to represent orbitals and arrows to represent electrons. a) aluminum, Z=13 b) phosphorus, Z# 15 c) bromine, Z-35 d) argon, Z = 18 Calendar To Do Notifications In Solved What are "valence electrons"? (b) What are - Chegg electrons"?(c) What does each box in an orbital diagram represent? (d) What quantity is represented by the direction (up or down) 01 the half-arrows in an orbital diagram? Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Chemistry and Biochemistry Flashcards - Quizlet Start studying Chemistry and Biochemistry. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
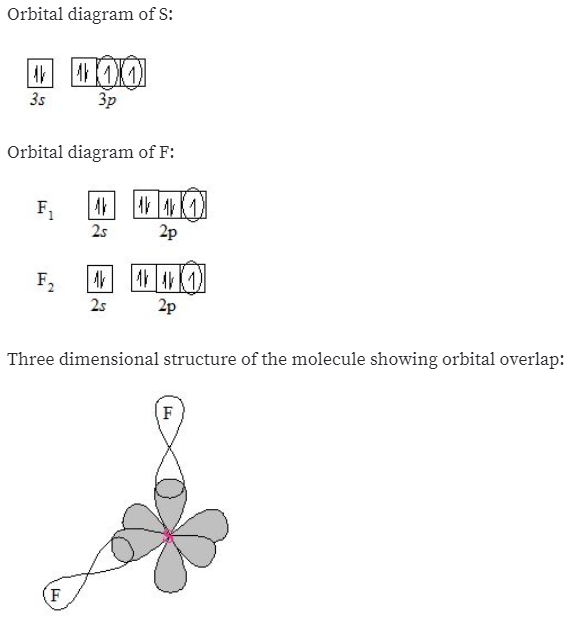
What does each box in an orbital diagram r... | Clutch Prep Recall that in an orbital diagram, boxes with arrow/ (s) are used to represent the electrons in an atom. There are 4 kinds of atomic orbitals, the s,p,d and f orbital: • s-subshell can hold a maximum of 2 electrons • p-subshell can hold a maximum of 6 electrons • d-subshell can hold a maximum of 10 electrons 92% (177 ratings) Answered: a) What are "valence electrons"? (b)… | bartleby Question. a) What are "valence electrons"? (b) What are "core electrons"? (c) What does each box in an orbital diagram represent? (d) What object is represented by the half arrows in. an orbital diagram? What does the direction of the arrow. signify? check_circle. Orbital Diagram For Selenium - schematron.org Selenium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. An orbital diagram uses boxes with arrows to represent the electrons in an atom. Each box in an The following is an orbital diagram for selenium. In writing an. Earth - Wikipedia Its axial tilt does undergo nutation; a slight, irregular motion with a main period of 18.6 years. The orientation (rather than the angle) of Earth's axis also changes over time, precessing around in a complete circle over each 25,800-year cycle; this precession is the reason for the difference between a sidereal year and a tropical year. Both ...
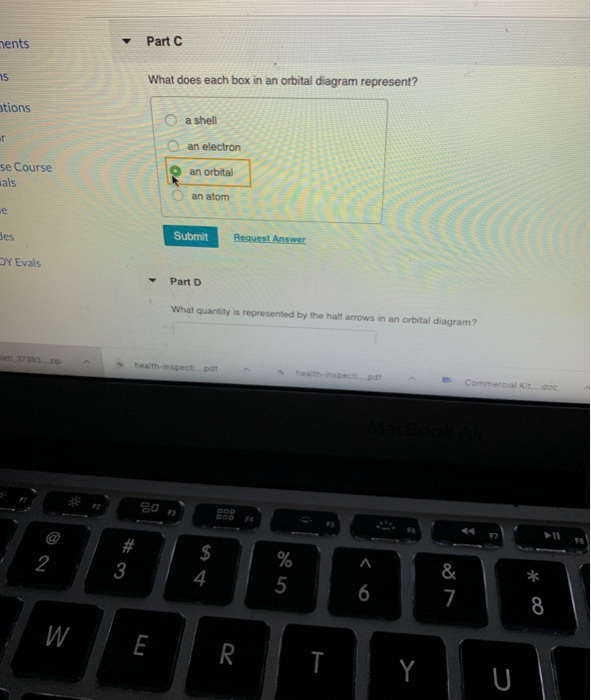
Solved ments Part C What does each box in an orbital ... 100% (4 ratings) Part C: Each box in the orbital …. View the full answer. Transcribed image text: ments Part C What does each box in an orbital diagram represent? ations a shell an electron an orbital se Course an atom Ses Submit Request Answer DY Evals Part D What quantity is represented by the half arrows in an orbital diagram? Previous ... Answered: What does each box in an orbital… | bartleby Solution for What does each box in an orbital diagram represent? close. Start your trial now! First week only $4.99! arrow_forward. learn. write. tutor. study resourcesexpand_more. Study Resources. We've got the study and writing resources you need for your assignments. ... PDF Iowa State University What does each box represent in an orbital diagram d.) What quantity is represented by the half arrows in an orbital diagram? An 3.) Write the electron configurations and condensed electron configurations for the following atoms, using the appropriate noble-gas core abbreviations for condensed configurations. Chemistry final Flashcards | Quizlet What does each box in an orbital diagram represent? Each box represents an orbital What quantity is represented by the half arrows in an orbital diagram? Each half arrow in an orbital diagram represents an electron. The direction of the half-arrow represents electron spin. Si- write its electron configuration Si: 1s^2 2s^2 2p^6 3s^2 sp^2
Lagrange point - Wikipedia The five Lagrange points are labelled and defined as follows: L 1 point. The L 1 point lies on the line defined between the two large masses M 1 and M 2.It is the point where the gravitational attraction of M 2 and that of M 1 combine to produce an equilibrium. An object that orbits the Sun more closely than Earth would normally have a shorter orbital period than Earth, but that …
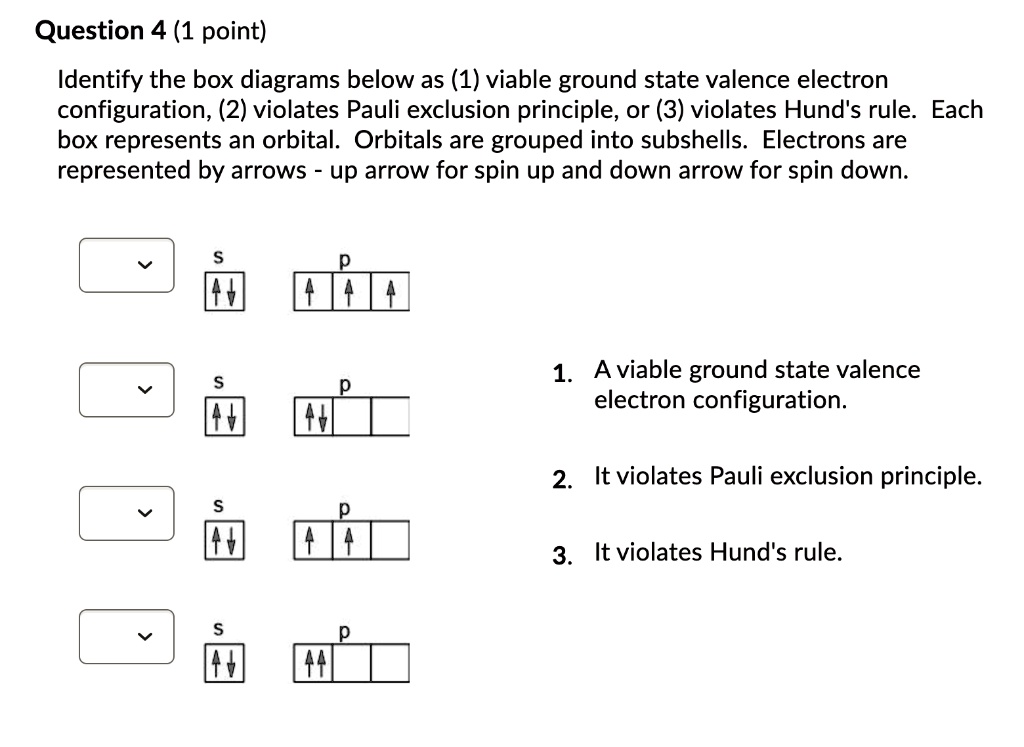
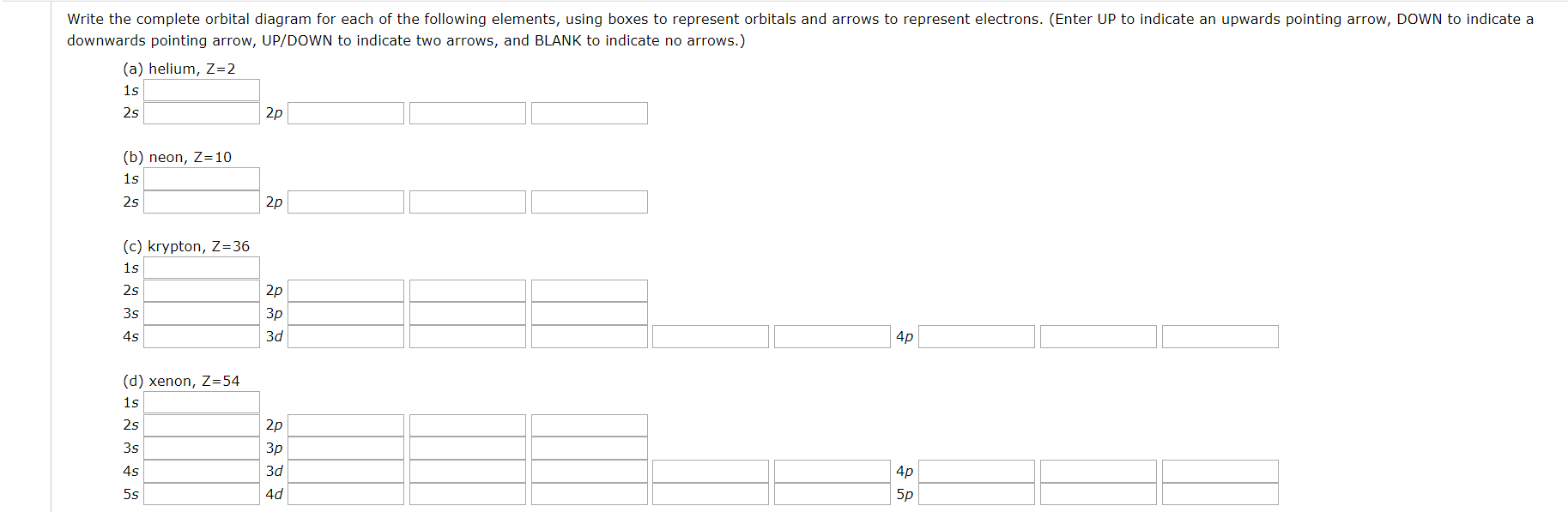
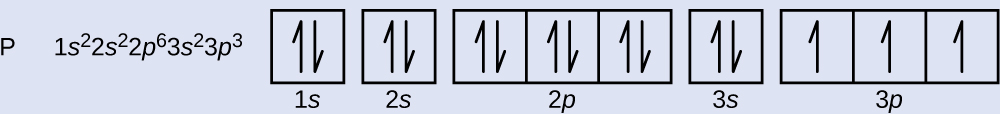
PDF Chapter 6: Electron Configurations c) What does each box in an orbital diagram represent? d) What quantity is represented by the direction (up or down) of the half-arrows in an orbital diagram? 2. What are the characteristics of Noble Gases? 3. For each element, count the number of valence electrons, core electrons, and unpaired electrons in the ground state: a) Carbon b) Phosphorus
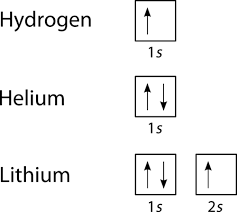
What does each box in an orbital diagram represent? Each box in an orbital diagram represents an orbital. Orbitals have a capacity of two electrons. Arrows are drawn inside the boxes to represent electrons. Two electrons in the same orbital must have opposite spin so the arrows are drawn pointing in opposite directions. Click to see full answer. Also know, what does an orbital diagram represent?
Hund's Rule and Orbital Filling Diagrams | Chemistry for ... Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
Chemistey Ex 2 Flashcards | Quizlet One electron must be placed in each orbital of equal (degenerate) energy before electrons are paired up. All unpaired electrons in a sublevel should have parallel spins. Hund's rule states the most stable configuration occurs when electrons occupy degenerate orbitals in such a way as to _____ the number of electrons with the same spin.
Chapter 5 Section 3 Flashcards - Quizlet an orbital with one electron. A box containing both up and down arrows represent. a filled orbital. What does Hund's rule state? 1. Every orbital in a sublevel is singly occupied before any orbital is doubly occupied. 2. All of the electrons in singly occupied orbitals have the same spin (to maximize total spin).
How do you use molecular orbital diagrams? Each box in an orbital diagram represents an orbital. Orbitals have a capacity of two electrons. Arrows are drawn inside the boxes to represent electrons. Two electrons in the same orbital must have opposite spin so the arrows are drawn pointing in opposite directions. Similar Asks. 28.
3.7 Orbital DIagrams Flashcards | Quizlet The orbital diagram provided represents what element? Report your answer using the chemical symbol for the element. O. In an orbital diagram, such as the one below, each small box represents which of the following? Select the correct answer below: a shell a subshell an individual orbital an individual electron.
Chapter 6, Electronic Structure of Atoms Video ... - Numerade The following diagrams represent two electromagnetic waves, drawn on the same scale. ... What does each box in an orbital diagram represent? (d) What object is represented by the half arrows in an orbital diagram? What does the direction of the arrow signify? KC Kevin C. ...
How do you do an orbital filling? Each box in an orbital diagram represents an orbital. Orbitals have a capacity of two electrons. Arrows are drawn inside the boxes to represent electrons. Two electrons in the same orbital must have opposite spin so the arrows are drawn pointing in opposite directions.
Answered: (a) What are "valence electrons"? (b)… | bartleby (a) What are "valence electrons"? (b) What are "core elec- trons"? (c) What does each box in an orbital diagram rep- resent? (d) What object is represented by the half arrows in an orbital diagram? What does the direction of the arrow signify?
What does each box in an orbital diagram represent ... What does each box in an orbital diagram represent? Orbital Diagrams: These are also known as electron-in-a-box diagrams. This is a simplified diagram of how the electrons are arranged within the...
Hund's Rule, the Pauli Exclusion Principle & the ... - Study.com Nov 05, 2021 · The box represents the orbital of the 1s subshell. The up arrows are electrons with spin-up, and the down arrows are electrons with spin-down. Only two electrons with different spins can occupy a ...
SOLVED:(a) What are "valence electrons"? (b ... - Numerade Call it an electron. This show number three. So you just like that? So the next question Axl, what's does each box in an opiate to diagram represents So, like, if we have the pr bitter say, Saxon, what's does this box this box is this Three boxes represent So once it means is this the boxes forever? Sainz No saw har bitters.
Electron Configuration Questions and Answers | Study.com Get help with your Electron configuration homework. Access the answers to hundreds of Electron configuration questions that are explained in a …
Optical Tables, 210 mm (8.3") Thick - Thorlabs May 06, 2019 · Thorlabs' 210 mm (8.3) thick Nexus® optical tables are offered in a variety of lengths and widths from 3' x 6' (1 m x 2 m) to 4.8' x 8' (1.5 m x 2.5 m). They feature all-steel construction, excellent thermal stability, and broadband damping optimized for each table size.
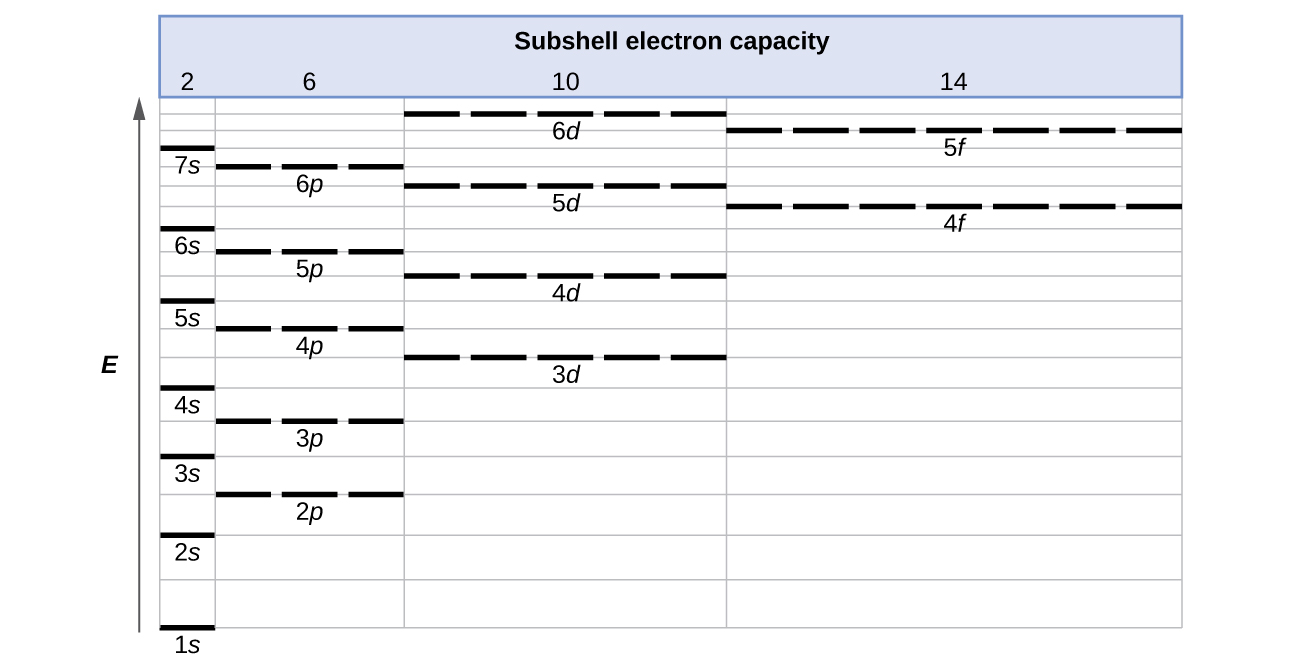
What do orbital diagrams show? - AskingLot.com Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. You jump up a little bit in energy and we get the 2s orbital that make it the 2p sublevel. What is the orbital diagram for chlorine?
What is the orbital notation for he ... Orbital diagrams use the same basic format, but instead of numbers for the electrons, they use ↑ and ↓ arrows, as well as giving each orbital its own line, to represent the spins of the electrons too. What do the arrows represent in an orbital diagram? An orbital diagram uses boxes with arrows to represent the electrons in an atom.
(PDF) University Physics Volume1-OP | Rimit ... - Academia.edu Academia.edu is a platform for academics to share research papers.
Learn About Box Diagram | Chegg.com Each box represents a single orbital and the number of spins in each box represents the number of electrons in that particular orbital. S-orbital can accommodate 2 electrons. P-orbital has three orientations and so can accommodate 6 electrons. D-orbital has 5 orientations and so can accommodate 10 electrons.
CHEMISTRY CIRRITO CHAPTER 5 Flashcards - Quizlet What does each small box in the diagram represent? the orbital. How many electrons can each orbital hold? 2. How many electrons can the d sublevel hold? 10. Which is associated with more energy: the 2s or the 2p orbital? 2p. Which energy level has the least amount of energy? 1s.

/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)





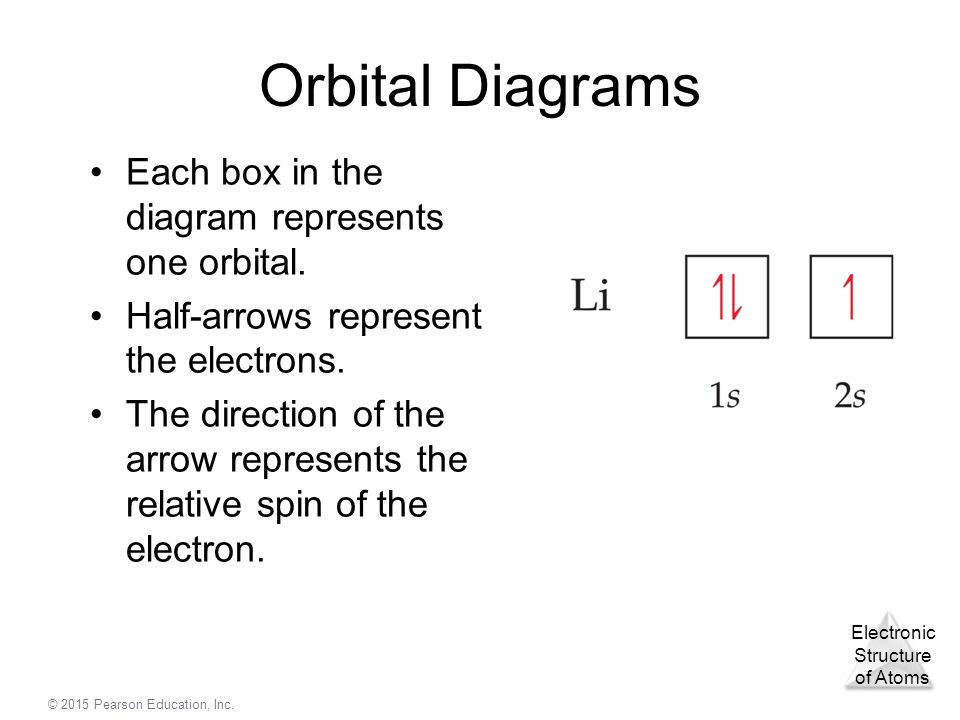
















0 Response to "40 what does each box in an orbital diagram represent"
Post a Comment