36 pauli exclusion principle diagram
Hund's Rule, Pauli Exclusion Principle & Orbitals Diagrams ... Hund's Rule, Pauli Exclusion Principle & Orbitals Diagrams. DRAFT. 10th - 11th grade . Played 0 times. 0% average accuracy. a few seconds ago by. lhugh4_47453. 0. Save. Edit. Edit. Hund's Rule, Pauli Exclusion Principle & Orbitals Diagrams DRAFT. a few seconds ago by. lhugh4_47453. 10th - 11th grade . Played 0 times. 0 likes. 0% average ... Pauli exclusion principle - Wikipedia The Pauli exclusion principle (German: Paulisches Ausschließungsprinzip) is the quantum mechanical principle which states that two or more identical fermions (particles with half-integer spin) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulated by Austrian physicist Wolfgang Pauli in 1925 for electrons, and later extended to all fermions ...
pauli exclusion principle Flashcards and Study Sets | Quizlet Aufbau Diagram, Hund's rule, and Pauli's exclusion Principle Aufbau's Principle Hund's Rule Pauli's Exclusion Principle Electrons fill energy levels and orbitals from the lowest ener… Electrons enter orbitals of equal energy one at a time to mini… Each orbital can hold two electrons with opposite spins (Only… 13 Terms GabPerfetti
Pauli exclusion principle diagram
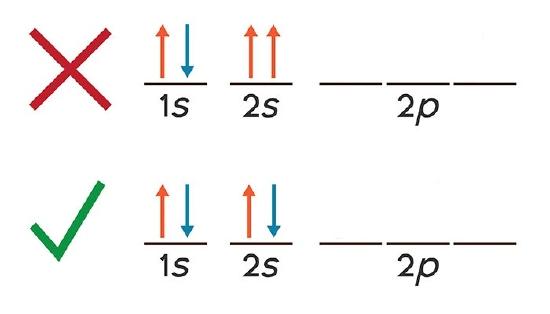
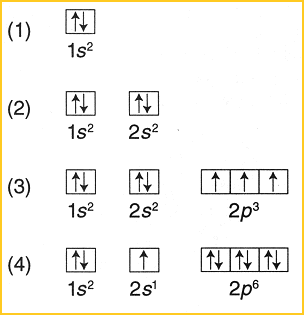
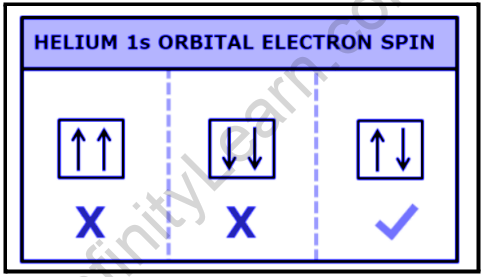
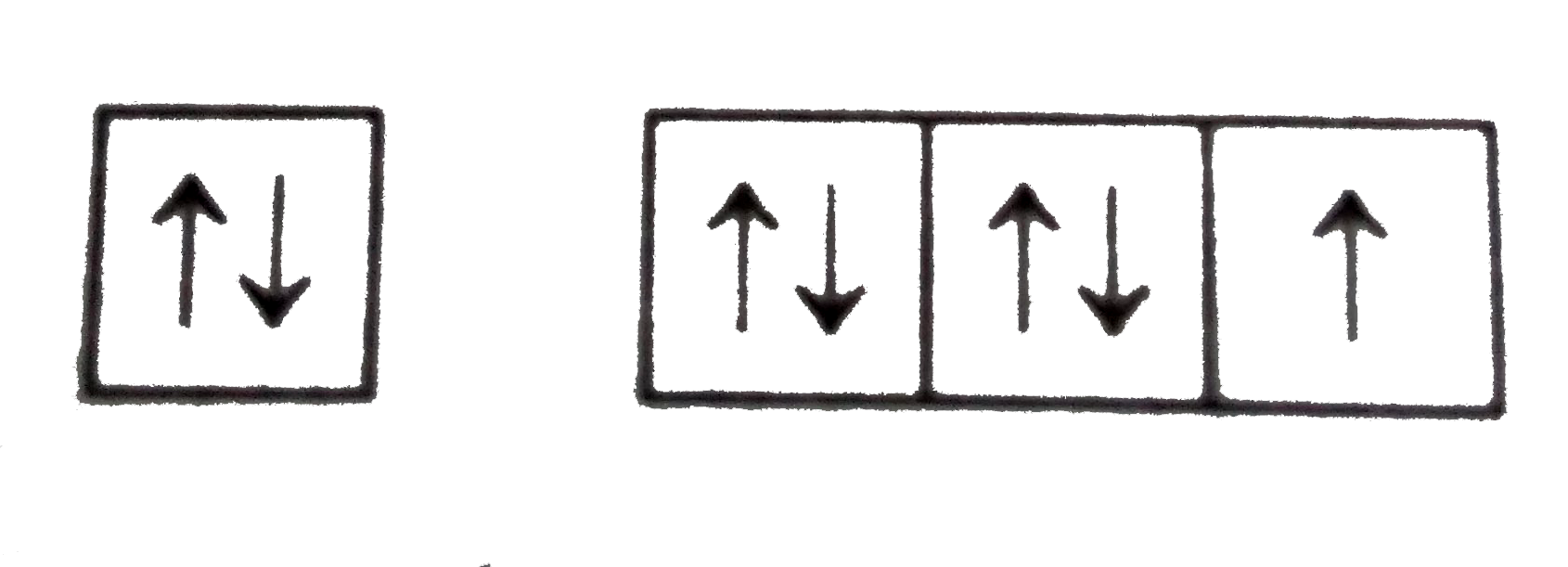
Which diagram shows electrons violating the Pauli ... Edufirst. Pauli exclusionj priniciple states that two electrons cannot have the same quantum state. These are valid representations: ⇅ ⇅ ↑. ⇅ ⇅ ↑ ↑ ↑. ⇅ ↑. This is not valid: ⇵ ↑↑ because it shows that the two last electrons occupy the same orbital with the same spin number, which means that those two electrons have ... What are Hund's Rule, Pauli Exclusion Principle, and ... Aufbau Principle: lower energy orbitals fill before higher energy orbitals. Hund's Rule: one electron goes into each until all of them are half full before pairing up. Pauli Exclusion Principle: no two electrons can be identified by the same set of quantum numbers (i.e. must have different spins). I'll start by explaining what each means, and then we'll talk about how they're related: The ... Pauli Exclusion Principle - ChemistryGod As we can see from the above diagram, all paired electrons in an orbital have opposite spins. It is the violation of the principle to place parallel spins in an orbital. The Pauli exclusion principle is fundamental to the structure of the atom. It affects the electronic configuration of the atom.
Pauli exclusion principle diagram. Hund's Rule, the Pauli Exclusion Principle & the Aufbau ... Pauli Exclusion Principle The box represents the orbital of the 1s subshell. The up arrows are electrons with spin-up, and the down arrows are electrons with spin-down. Only two electrons with... Solved: Which of the following orbital diagrams are ... 773-8-41PP SA: 5350. SR: 9877. According to Pauli Exclusion Principle, no two electrons in an atom can have the same four quantum number Pauli Exclusion Principle | definition and example According to Pauli Exclusion Principle, No two electrons can have samples values of the quantum numbers n, l,m l and m s or they can not have all these quantum numbers similar; at least one of the four numbers which are strictly required to Specify the state of an electron should be different from the quantum numbers which specify the state of another electron of the same atom. Pauli Exclusion Principle Orbital Diagram - The 41 Best ... Pauli Exclusion Principle Orbital Diagram Images, posts & videos related to "Pauli Exclusion Principle Orbital Diagram" Forgive me if I say or phrase this wrong it is regarding why the Pauli Exclusion principle governs the rule only spin up and spin down each orbital s,p,d,f etccc.
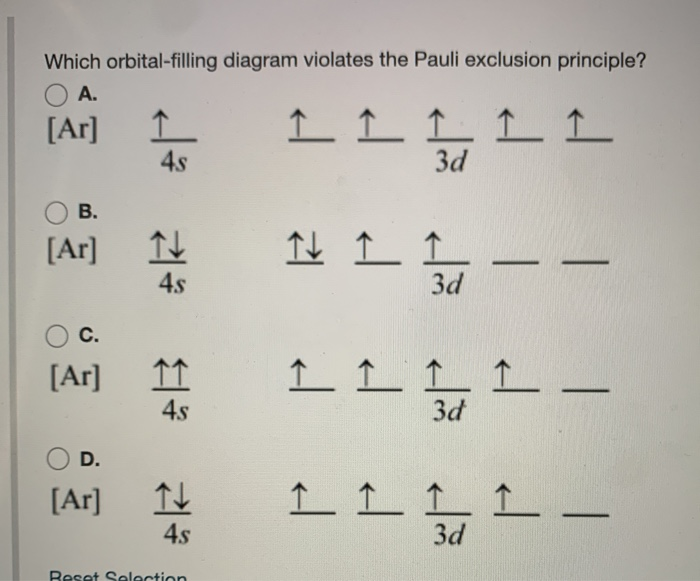
Solved Which orbital-filling diagram violates the Pauli ... Best Answer. This is the best answer based on feedback and ratings. 100% (14 ratings) as per Pauli's Exclusion P [ …. View the full answer. Transcribed image text: Which orbital-filling diagram violates the Pauli exclusion principle? Previous question Next question. Pauli Exclusion Principle | Chemistry for Non-Majors So the two electrons in the 1s orbital are each unique and distinct from one another because their spins are different. This observation leads to the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of four quantum numbers.The energy of the electron is specified by the principal, angular momentum, and magnetic quantum numbers. Pauli Exclusion Principle - Meaning, Applications, and FAQs The Pauli exclusion principle in chemistry is as important as the Pauli exclusion principle in physics. Pauli Exclusion Principle in Chemistry: In chemistry, the principle is mainly used to explain or determine the electron shell structure of atoms and predict which atoms are likely to have free electrons at the end of configurations. Pauli Exclusion Principle - Chemistry LibreTexts Pauli Exclusion Principle. The Pauli Exclusion Principle states that, in an atom or molecule, no two electrons can have the same four electronic quantum numbers. As an orbital can contain a maximum of only two electrons, the two electrons must have opposing spins. This means if one electron is assigned as a spin up (+1/2) electron, the other ...
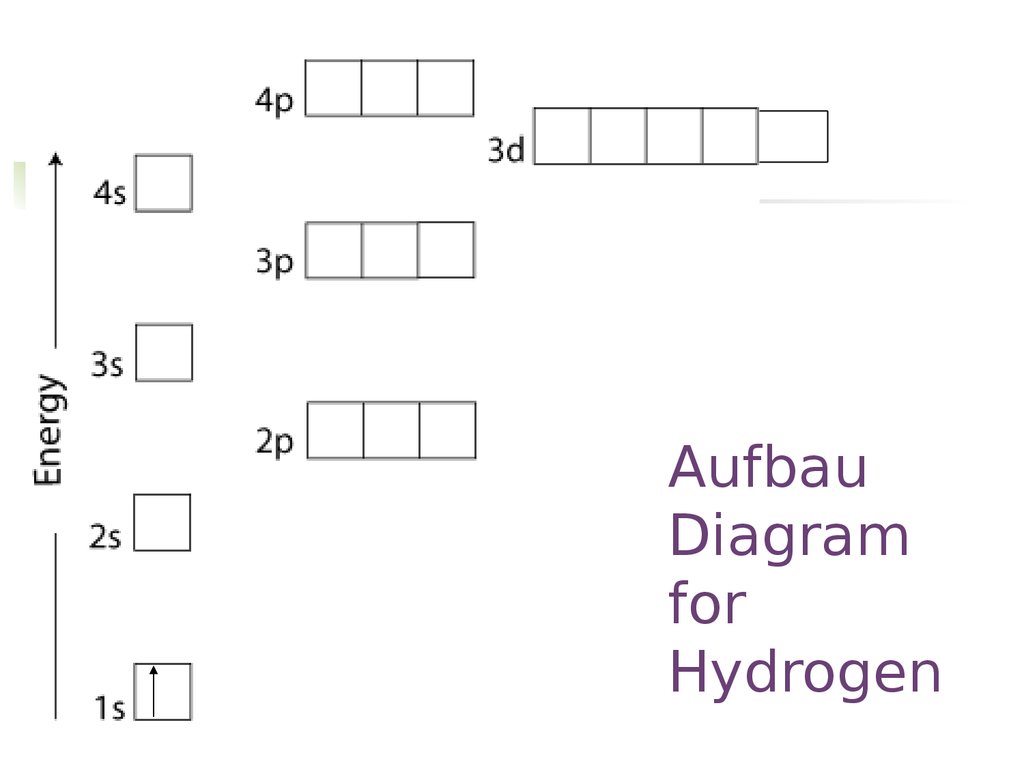
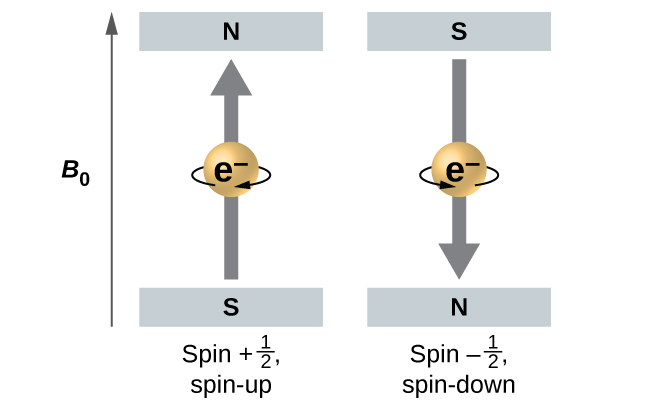
How is the Pauli exclusion principle different from the ... The Pauli Exclusion Principle states that, in an atom or molecule, no two electrons can have the same four electronic quantum numbers. Electrons in the same orbital have the same first three quantum numbers, e.g., n=1n=1, l=0l=0, ml=0ml=0 for the 1s subshell. What are Hund's Rule, Pauli Exclusion Principle, and the ... Pauli Exclusion Principle. Pauli exclusion principle states that in a single atom no two electrons will have an identical set or the same quantum numbers (n, l, ml, and ms). To put it in simple terms, every electron should have or be in its own unique state (singlet state). There are two salient rules that the Pauli Exclusion Principle follows: 7.12: The Pauli Exclusion Principle - The Pauli Exclusion ... The orbital diagram of hydrogen, therefore, has one upward arrow. The electron configuration of helium is 1s 2. The two electrons have three identical quantum numbers, as they belong to the same shell and subshell. Their spin quantum numbers are different, in accordance with the Pauli exclusion principle. Pauli Exclusion Principle: Definition, Application ... Feb 16, 2022 · Pauli exclusion principle is a fundamental principle along with Aufbau’s Principle and Hund’s Rule in chemistry. In the year 1925, the Austrian physicist Wolfgang Pauli proposed Pauli’s exclusion principle. It states that no two electrons in the same atom can possess identical values for all four of their quantum numbers.
Energy Levels, Energy Sublevels, Orbitals, & Pauli ... Energy Levels, Energy Sublevels, Orbitals, & Pauli Exclusion Principle.Chemistry Lecture #21.For a pdf transcript of this lecture, go to
What is the Pauli Exclusion Principle? + Example The Pauli Exclusion Principle states that no two electrons can have the same four quantum numbers. The fourth quantum number is the electron spin quantum number m_s=+-1/2. An orbital can contain a maximum of two electrons, which can have three quantum numbers in common, but not the same spin quantum number. One must spin up, +1/2, and the other must spin down, -1/2.
What are the Pauli Exclusion Principle, Aufbau Principle, and ... What are the Pauli Exclusion Principle, Aufbau Principle, and Hunds Rule? They are rules we use to fill electron orbital filling diagrams. Fill from the bo...
Pauli Exclusion Principle - an overview | ScienceDirect Topics Pauli's Exclusion Principle states that no two electrons in the same atom can have identical values for all four of their quantum numbers. In other words, (1) no more than two electrons can occupy the same orbital and (2) two electrons in the same orbital must have opposite spins ( Figure 46 (i) and (ii) ). Sign in to download full-size image
Pauli Exclusion Principle - Definition, Explanation, Examples The Pauli exclusion principle states that in a single atom no two electrons will have an identical set or the same quantum numbers (n, l, m , and m s ). To put it in simple terms, every electron should have or be in its own unique state (singlet state). There are two salient rules that the Pauli Exclusion Principle follows:
(PDF) Pauli Exclusion Principle - ResearchGate By the Pauli exclusion principle, each of the states contains zero or one electron. [ 68 ] Electrical conductivity takes place because of the presence of electrons in the states that are delocalized.
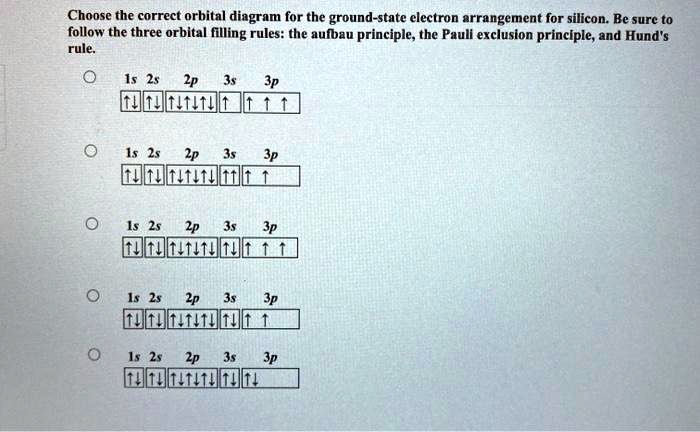
SOLVED:Apply the Pauli exclusion principle, the aufbau ... Apply the Pauli exclusion principle, the aufbau principle, and Hund's rule to write out the electron configuration and draw the orbital diagram for each of the following elements. a. silicon $\quad$ b. fluorine $\quad$ c. calcium $\quad$ d. krypton
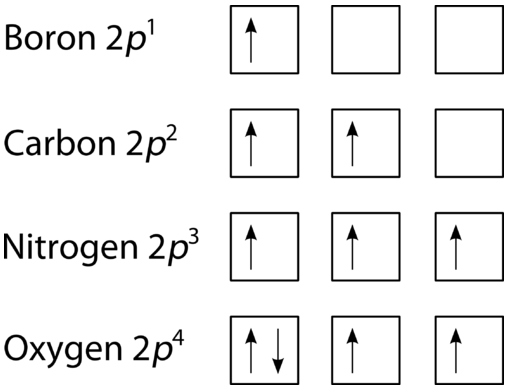
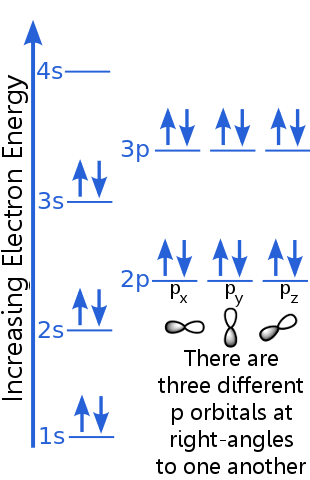
PDF Pauli, Hund and Aufbau The Periodic Table Pauli Exclusion Principle: No two electrons can have the same 4 quantum numbers. Hund's Rule (one of three) For an electron shell with multiple orbitals, the term with maximum number of unpaired spins has the lowest energy. There are exceptions to Aufbau principle and Hund's Rules, but not the Pauli exclusion principle
Which diagram shows electrons violating the Pauli ... 3rd diagram shows electrons violating the Pauli exclusion principle, as in that two electrons with same spin is present.. What is Pauli exclusive principle? Pauli exclusive principle tells about the orientation of electron inside the orbital as two electrons with different spin will present in an orbital and, it also said that no electron will have same value of four quantum number i.e. n, l ...
Hund's Rule, Pauli Exclusion Principle & Orbitals Diagrams ... An electron occupies the lowest energy orbital that can receive it. Q. All orbitals of equal energy are occupied by one electron before any single orbital is occupied by a second electron. Q. No two electrons in the same atom can have the same four quantum numbers. Q. Which guideline, Hund's rule or the Pauli exclusion principle, is violated ...
Pauli Exclusion Principle and Hund's Rule - Kentchemistry.com Pauli Exclusion Principle An orbital can hold 0, 1, or 2 electrons only, and if there are two electrons in the orbital, they must have opposite (paired) spins. When we draw electrons, we use up and down arrows. So, if an electron is paired up in a box, one arrow is up and the second must be down.
Pauli, Aufbau, Hund - ChemistNate b) violates the Aufbau principle, because the 1s orbital is missing an electron. There should not be electrons in 2s until 1s is filled! c) violates the Pauli Exclusion Principle since both electrons in the 1s orbital are "spin up". This means that both have the same m s and so they have the same four quantum numbers.
Pauli Exclusion Principle - ChemistryGod As we can see from the above diagram, all paired electrons in an orbital have opposite spins. It is the violation of the principle to place parallel spins in an orbital. The Pauli exclusion principle is fundamental to the structure of the atom. It affects the electronic configuration of the atom.
What are Hund's Rule, Pauli Exclusion Principle, and ... Aufbau Principle: lower energy orbitals fill before higher energy orbitals. Hund's Rule: one electron goes into each until all of them are half full before pairing up. Pauli Exclusion Principle: no two electrons can be identified by the same set of quantum numbers (i.e. must have different spins). I'll start by explaining what each means, and then we'll talk about how they're related: The ...
Which diagram shows electrons violating the Pauli ... Edufirst. Pauli exclusionj priniciple states that two electrons cannot have the same quantum state. These are valid representations: ⇅ ⇅ ↑. ⇅ ⇅ ↑ ↑ ↑. ⇅ ↑. This is not valid: ⇵ ↑↑ because it shows that the two last electrons occupy the same orbital with the same spin number, which means that those two electrons have ...




















0 Response to "36 pauli exclusion principle diagram"
Post a Comment