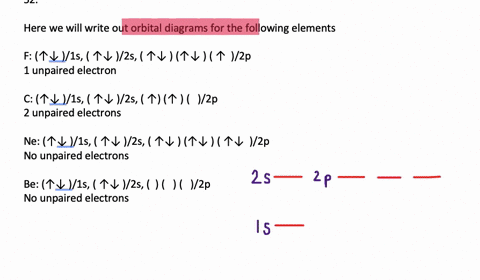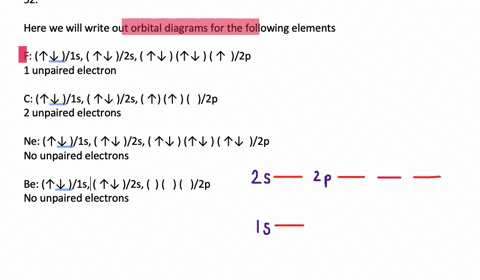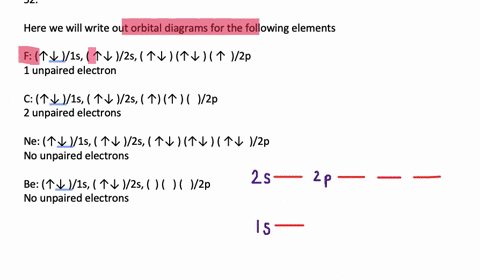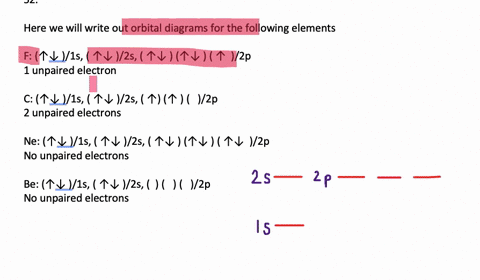
39 write orbital diagram for au+.
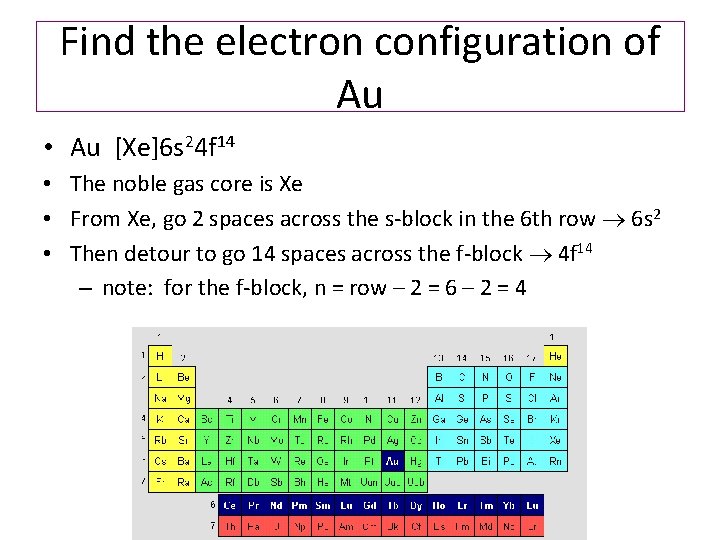
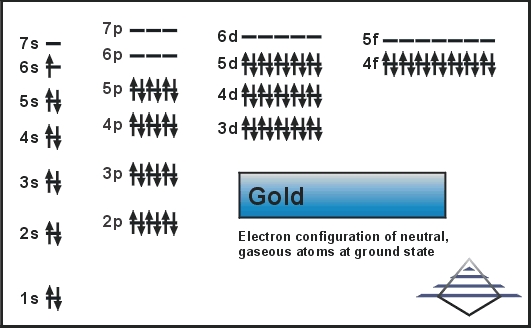
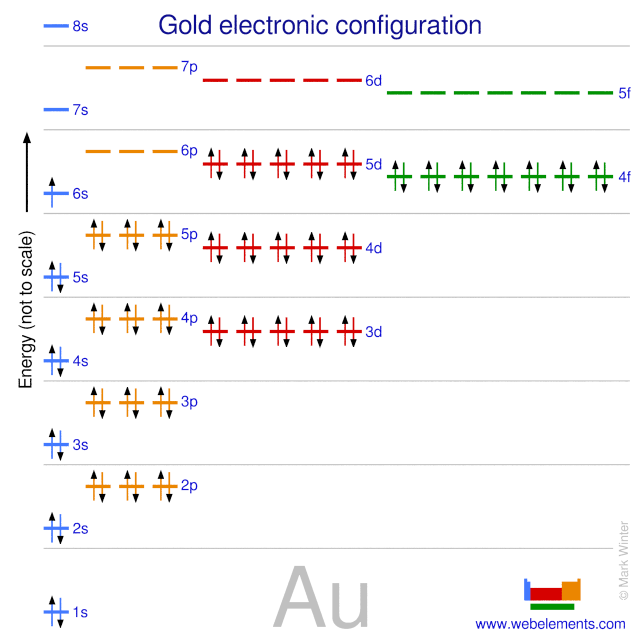
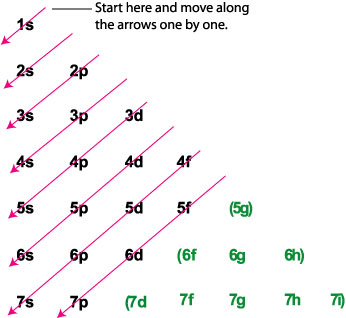
What is the orbital diagram for Au? - Answers The orbital diagram for gold starts with the base [Xe], which is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6. The outer shells are 6s2 5d9. Orbital Diagram For Au+ - schematron.org Write orbital diagram for Au+? (1) Which of the following clusters of orbitals would form the shape trigonal bipyramidal and would also be possible within the.Write the orbital diagram for Au+ Get Answer. Recently Asked Questions Five thousand bonds with a face value of $ each, are sold at The entry to record the issuance is a-Cash Discount on ...
Write orbital diagram for Au+? Jun 24, 2021 · Since Au+ has lost one electron and the numbers (or arrows in the diagram) represent the electrons, its configuration is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10. So there will not be the 6s orbital. Since all of the orbitals are filled with electrons (i.e., there are no unpaired electrons in Au+ orbital diagram), then the ion ...

Write orbital diagram for au+.
SOLVED:Write orbital diagrams for each ion and determine ... Problem 78 Hard Difficulty. Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+ Write orbital diagram for Au+? - Soetrust Feb 27, 2022 · Write orbital diagram for Au+? HERE THE ANSWERS. orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. you just have to fill the “boxes” with arrows, s orbital has only one box with two arrows; p has 3orbitals with 6arrows; d has 5 boxes with 10 arrows and f has 7boxes with 14 arrows. what is the orbital diagram for Au+, how do you fit the f ... How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 subshell? True or False 1.The contour of the orbital would extend further out along the x and y axes. 2. The value of ℓ would increase by . chemistry. How would the 2s and 3p orbitals differ from the 1s and 2p orbitals?
Write orbital diagram for au+.. (Get Answer) - Write orbital diagram for Au+. Determine if ... Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. Posted one year ago. Q: Write the abbreviated electron configuration and construct the orbital diagram for the chromium(II) ion. Is the ion paramagnetic or diamagnetic? ... Enter The Orbital Diagram For The Ion Au+., Write Orbital ... So for scandium the 1st and 2nd electron must be in 1s orbital, the 3rd and 4th in the 2s, the 5th through 10th in the 2p orbitals, etc. You are watching: Enter the orbital diagram for the ion au+. This is a memory device to remember the order of orbitals for the first two quantum numbers. Follow the arrow starting in the upper right, when the ... Write orbital diagram for Au+? - TheBasicAnswers.com May 20, 2021 · Since Au+ has lost one electron and the numbers (or arrows in the diagram) represent the electrons, its configuration is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10. So there will not be the 6s orbital. Write orbital diagrams for each ion and in... | Clutch Prep Problem: Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ FREE Expert Solution We're being asked to classify each ion as diamagnetic or paramagnetic. Recall that for: • diamagnetic: all of the electrons are paired • paramagnetic: at least one electron is unpaired.
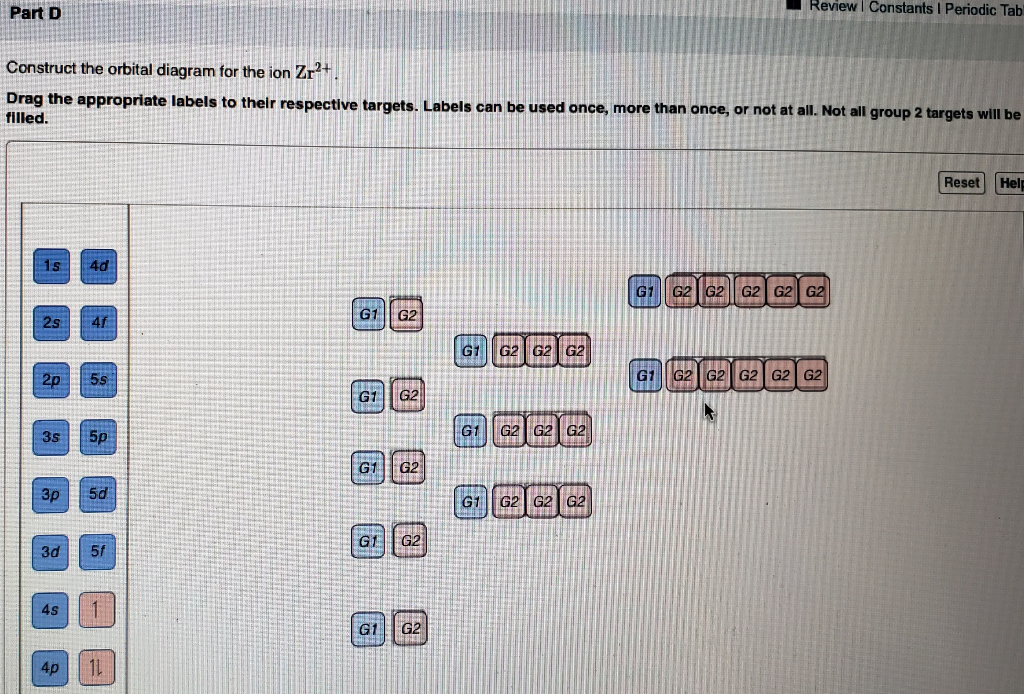
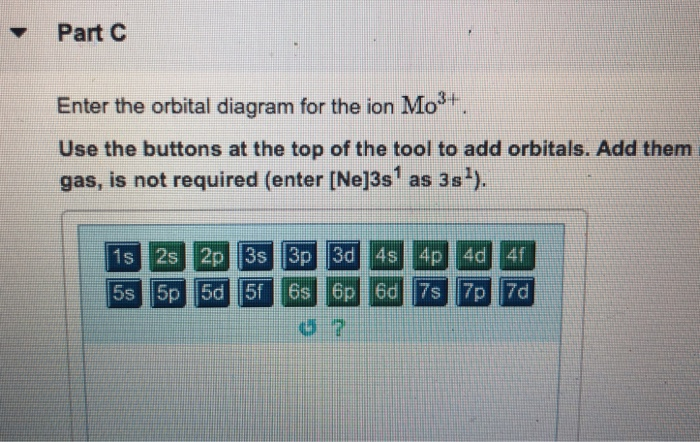
Solved A) Write orbital diagram for Au+. B) Write orbital ... A) Write orbital diagram for Au+. B) Write orbital diagram for Zr2+. Enter The Orbital Diagram For The Ion Mo3+. Answer to Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing.write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd 2+ diagramweb.net + diagramweb.net 3+ d. Zr 2+ Provide your answer: example:paramagnetic, diamagnetic, etc., . Orbital Diagram Au+ the atomic number of au is therefore, its for au+, one electron is removed from the outermost 6s orbital, making the configuration. orbital diagram for au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes" with arrows, s orbital.orbital diagrams of atoms diagram shows how the electrons are distributed … Enter the orbital diagram for the ion au+. The number of unpaired electrons for any free atoms or ions can be determined by using the orbital diagram. An orbital diagram is the pictorial representation of shells in an atom by using square boxes (one box for s-orbital, three boxes for p-orbitals, five boxes for d-orbitals and seven boxes for f-orbitals) those boxes are filled by electrons using the following principles: Aufbau principle ...
What is the electron configuration of Au+? | Socratic [Xe] 4f^14 5d^10 The atomic number of Au is 79. Therefore, its configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 4f^14 5d^10 6s^1 or, [Xe] 4f^14 5d^10 6s^1 For Au^+, one electron is removed from the outermost 6s orbital, making the configuration, [Xe] 4f^14 5d^10 Solved Write orbital diagram for Au+. Determine if the ion ... This problem has been solved! Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... Orbital Diagram For Au+ - Wiring Diagrams How to find the isotope of. Answer to Write orbital diagram for Au+. Draw an Molecular Orbital energy diagram and predict the bond order of L 2. Using a partial orbital diagram, show . Electron Configuration Electron Configuration Watch later Watch on
(Get Answer) - Write orbital diagram for Au + .. Write ... Write orbital diagram for Au + . Write orbital diagram for Au+. Jan 11 2022 08:18 AM. Solution.pdf.
what is the orbital diagram for Au+, how do you fit the f ... How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 subshell? True or False 1.The contour of the orbital would extend further out along the x and y axes. 2. The value of ℓ would increase by . chemistry. How would the 2s and 3p orbitals differ from the 1s and 2p orbitals?
Write orbital diagram for Au+? - Soetrust Feb 27, 2022 · Write orbital diagram for Au+? HERE THE ANSWERS. orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. you just have to fill the “boxes” with arrows, s orbital has only one box with two arrows; p has 3orbitals with 6arrows; d has 5 boxes with 10 arrows and f has 7boxes with 14 arrows.
SOLVED:Write orbital diagrams for each ion and determine ... Problem 78 Hard Difficulty. Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+


















0 Response to "39 write orbital diagram for au+."
Post a Comment