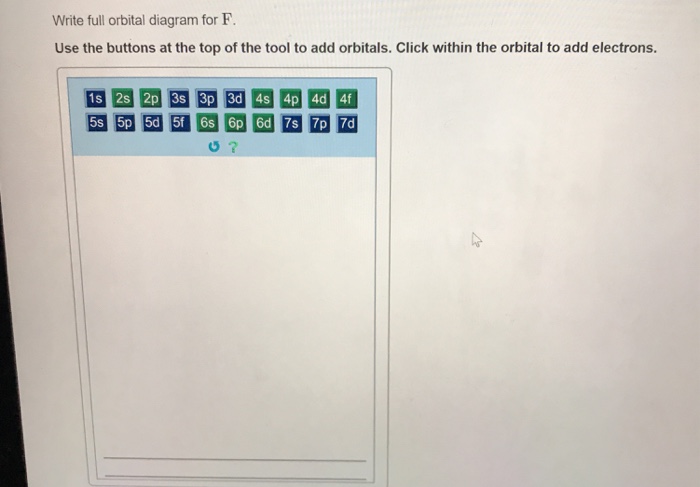
38 write full orbital diagram for f
PLEASE HELP Write the full electronic configuration and ... PLEASE HELP Write the full electronic configuration and draw the orbital box diagram of iron in its oxidation state in K3 Fe(CN)6. Hence determine the number of unpaired electrons in this state. Neon(Ne) electron configuration and orbital diagram Neon (Ne) atom electron configuration (Bohr model) K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n 2. For example, n = 1 for K orbit. The electron holding capacity of K orbit is 2n 2 = 2 × 1 2 = 2 electrons.
PDF Electron Configurations and Orbital Diagrams key Electron Configurations and Orbital Diagrams KEY Draw orbital diagrams for the following elements: 1. phosphorus ... Write the electron configuration (full, and in core notation) for the following ions: 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8.
Write full orbital diagram for f
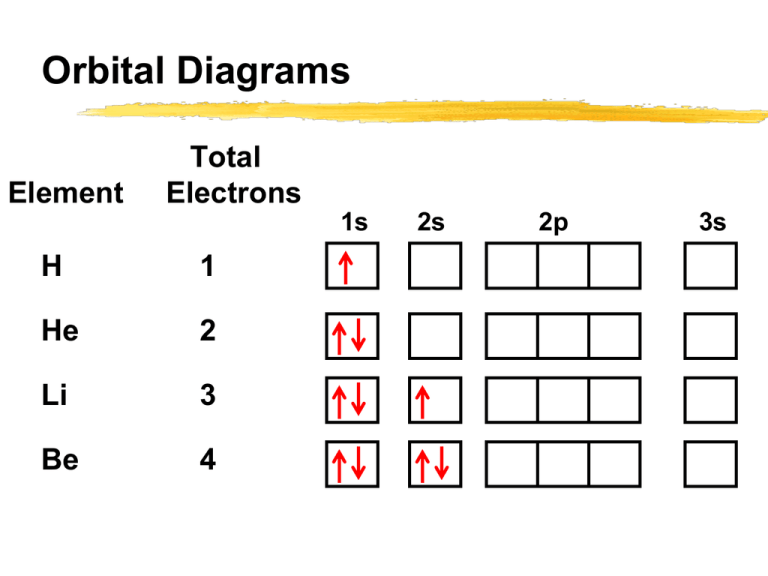
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Electron Configurations - Department of Chemistry ... Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements.
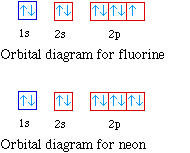
Write full orbital diagram for f. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... Orbitals Chemistry (Shapes of Atomic Orbitals) - Shape of ... Orbitals Chemistry (s, p, d, and f Orbital) - Atomic Orbitals are of four different kinds, denoted s, p, d, and f, each with a different shape. Of the four, we'll be concerned primarily with s and p orbitals because these are the most common in organic chemistry. Learn more about atomic orbital at Byjus SOLVED:Write full orbital diagrams and indicate the number ... Problem 52 Medium Difficulty. Write full orbital diagrams and indicate the number of unpaired electrons for each element. (a) $\mathrm{F}$ (b) C (c) Ne (d) Be Complete An Orbital Diagram For Scandium (sc). Use this tool to draw the orbital diagram. 3d. 4p. Draw orbital diagrams for the following elements. Write the electron configuration (full, and in core notation). 1. scandium. ↑↓. ↑↓. ↑↓ ↑↓ ↑↓. ↑↓. Contrary to what you may have seen, for Sc and the remaining elements, the 4s is not lower in energy than the 3d. In fact ...
Part A Write full orbital diagram for Use the buttons at ... Correct Part F Indicate the number of unpaired electrons in it. Express your answer as an integer. ANSWER: ANSWER: = 0 Correct Part G Write full orbital diagram for. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Orbital Diagram For Strontium - schematron.org In an orbital (box) diagram a box represents each notation and an orbital diagram. strontium atom (a) in the spdf notation and (b) in the. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of strontium (atomic number: 38), the most. Solved Write the full orbital diagram for F. Drag the ... Transcribed image text: Write the full orbital diagram for F. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. TL 1s 11 11 11 1 1 1s 2s 25 2p 2p Part B Indicate the number of unpaired electrons in F. Express your answer as an integer. Answered: For an atom of 9F, write its: Full… | bartleby For an atom of 9F, write its: Full electron configuration: 1s2_____ Draw a full orbital diagram (e¯s as arrows in boxes with labels): Count the unpaired e¯s _____ Þ 9F atom is diamagnetic ? paramagnetic (mark one) Write four quantum numbers n, l, ml, ms separately for each of the valence e¯s in the configuration: _________. Write four ...
Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s22s22p3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital. How do you write the orbital diagram for carbon? | Socratic The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ... What Are The 3 Rules For Orbital Diagrams Orbital diagrams are like the configuration notation just introduced, except with the spins of electrons indicated. This means that when writing orbital diagrams for partially full shells, fill in all of the up-spin electrons before adding any down-spin electrons. … Solved Part A Write full orbital diagram for F. Drag the ... Transcribed image text: Part A Write full orbital diagram for F. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Reset Help @@@BE G1G1GT Submit Request Answer - Part B Indicate the number of unpaired electrons in F. Express your answer as an integer.
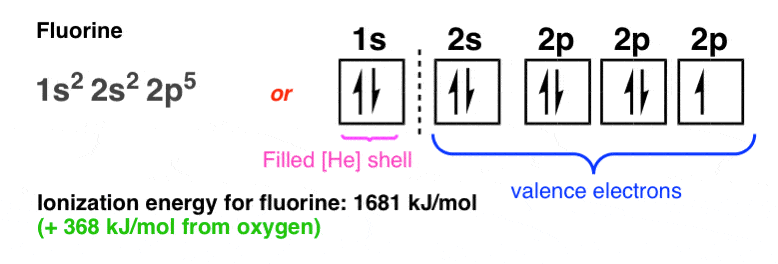
61 Tros Introductory Chemistry Chapt PracticeWrite the ... 63 Tro's "Introductory Chemistry", Chapt Valence Electrons • The electrons in all the subshells with the highest principal energy shells are called the valence electrons. • Electrons in lower energy shells are called core electrons. • Chemists have observed that one of the most important factors in the way an atom behaves, both chemically and physically, is the number of valence electrons.
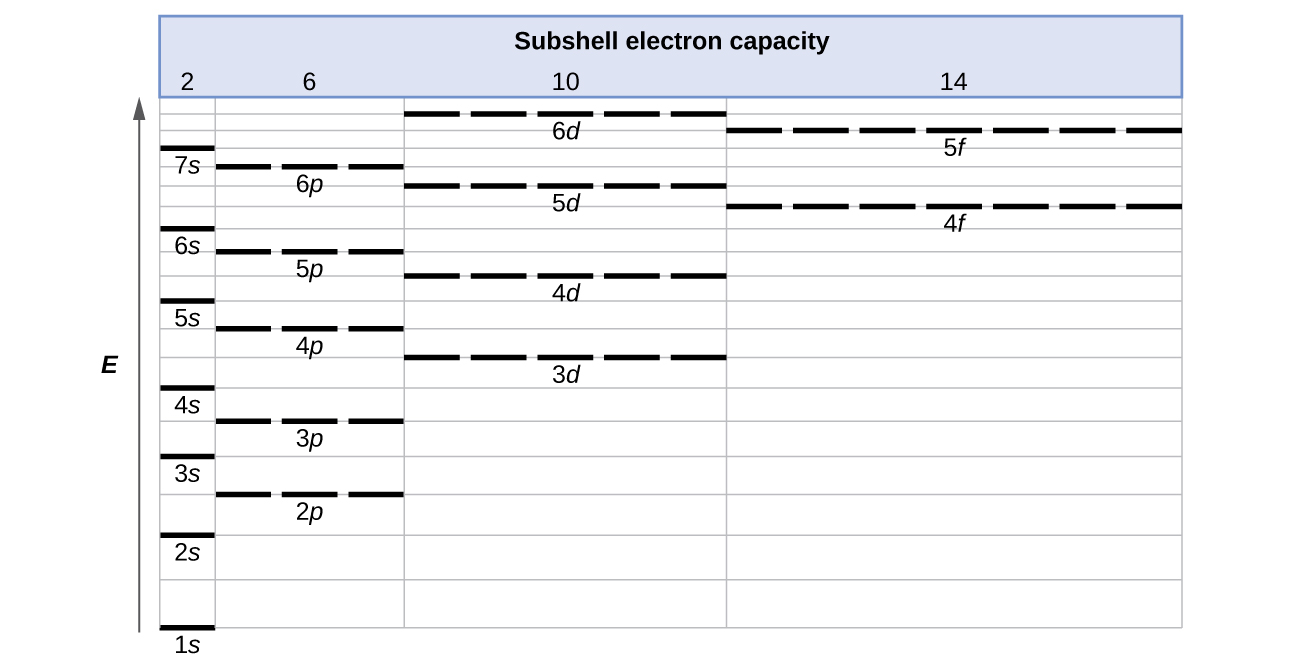
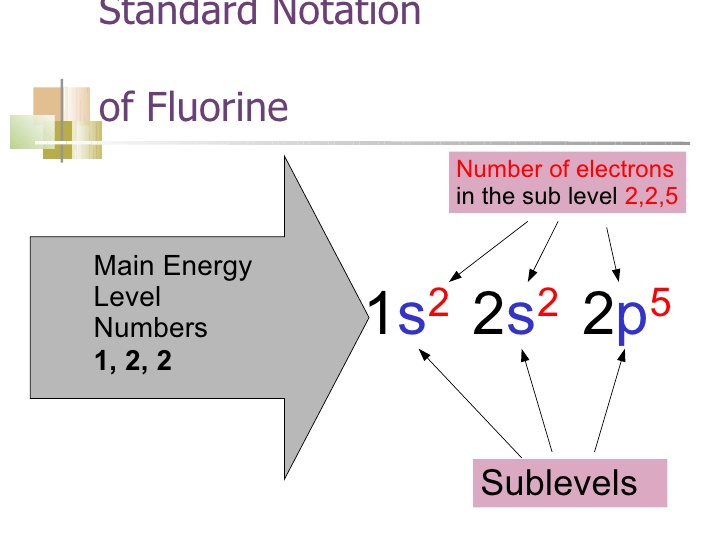
How to Do Orbital Diagrams - Sciencing The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ...
Write the full orbital diagram for each element. a. S, b ... Find step-by-step Chemistry solutions and your answer to the following textbook question: Write the full orbital diagram for each element. a. S, b. Ca, c. Ne, d. He.
SOLVED:Write the full orbital diagram for each element. a ... SOLVED:Write the full orbital diagram for each element. a. N b. F c. Mg d. Al. Problem. Write the full orbital diagram for each element. ….
Answered: write the electron configuration… | bartleby s orbital b. p orbital c. d orbital d. f orbital arrow_forward The complete orbital notation diagram of an atom is shown. based on the diagram what values can be assigned to the principal quantum number dor the elctrons in the atom what information does this quantum number provide about the location of the electron
Chapter 8 Chemistry Flashcards | Quizlet An orbital diagram is a different way to show the electron configuration of an atom. It symbolizes the electron as an arrow in a box that represents the orbital. The orbital for a hydrogen atom: (is a box with 1s under it and an H next to it. It has one arrow in it facing up)
Fluorine(F) electron configuration and orbital diagram To write the orbital diagram of fluorine (F), you have to do the electron configuration of fluorine. Which has been discussed in detail above. Fluorine (F) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital.
How to Write the Electron Configuration for Calcium (Ca) In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Electron Configuration for Fluorine (F) - UMD In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. The remaining five electrons will go in the 2p orbital. Therefore the F electron configuration will be 1s 2 2s 2 2p 5.
(a) Write the full orbital diagram for F. (b) Indicate the ... Answer to: (a) Write the full orbital diagram for F. (b) Indicate the number of unpaired electrons in it. By signing up, you'll get thousands of...
Electron Configurations - Department of Chemistry ... Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements.
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
















![Electron Configuration | Chemistry [Master]](https://textimgs.s3.amazonaws.com/boundless-chemistry/agram-carbon-hund-27s-rule.svg)









0 Response to "38 write full orbital diagram for f"
Post a Comment