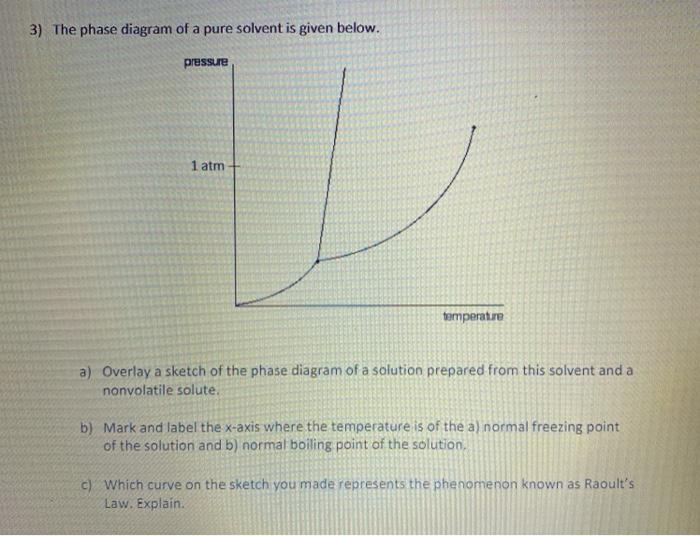
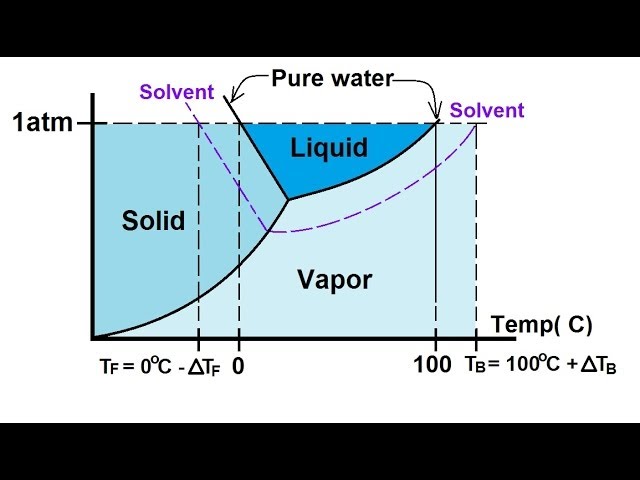
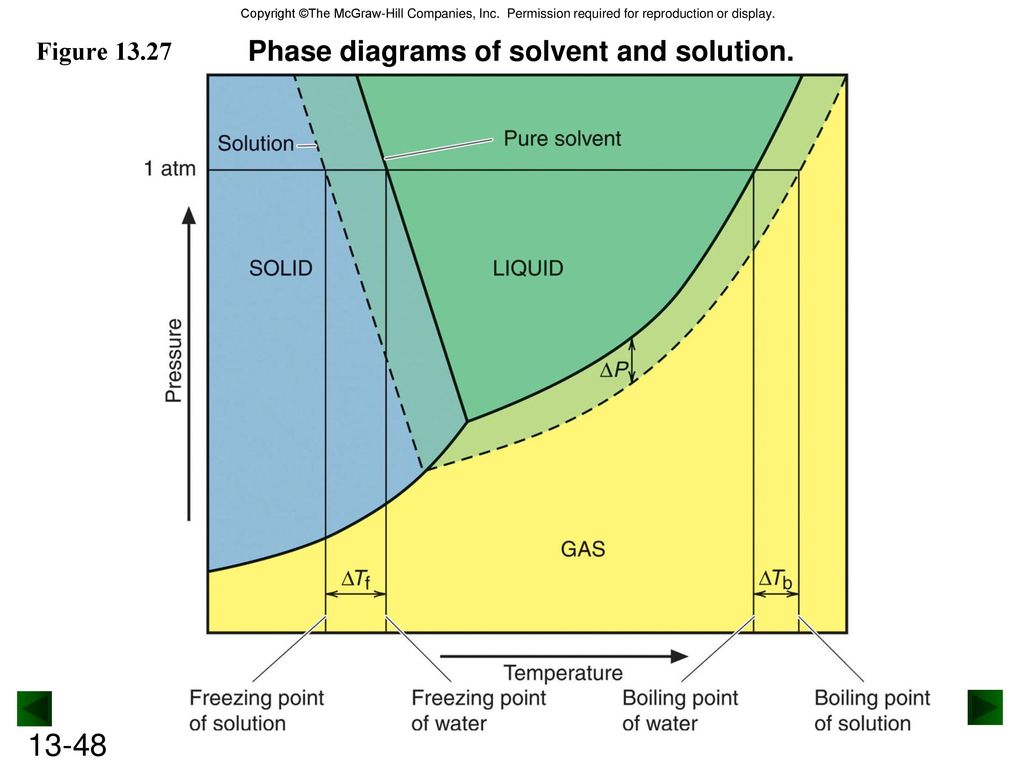
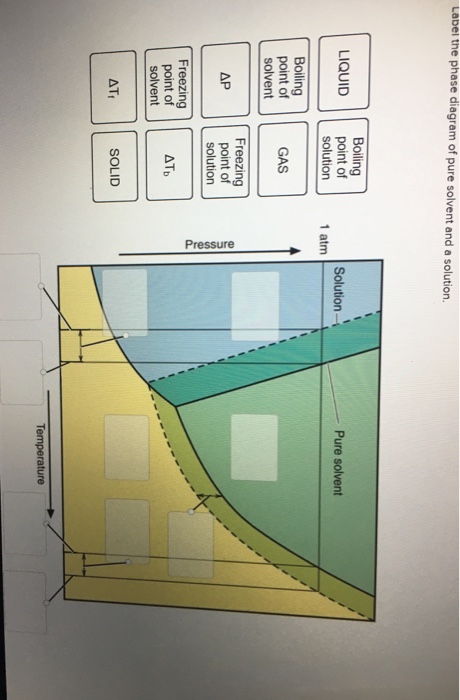
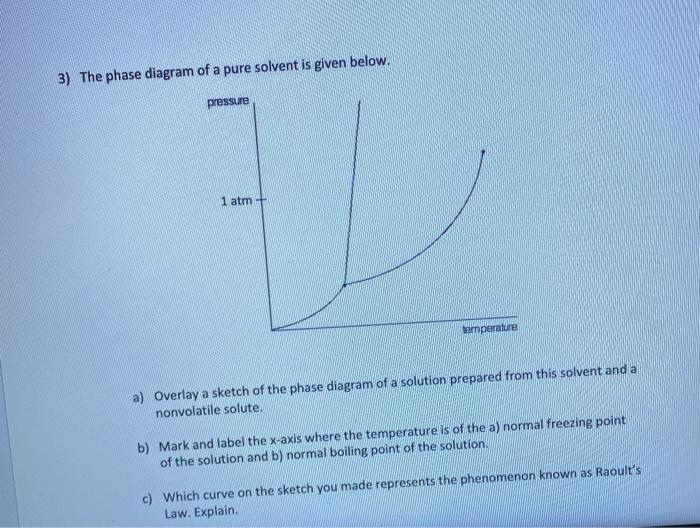
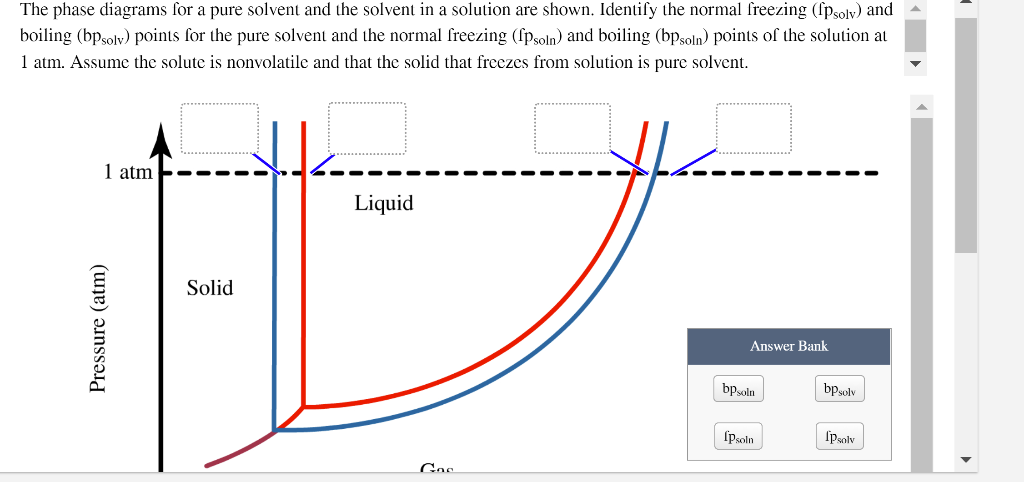
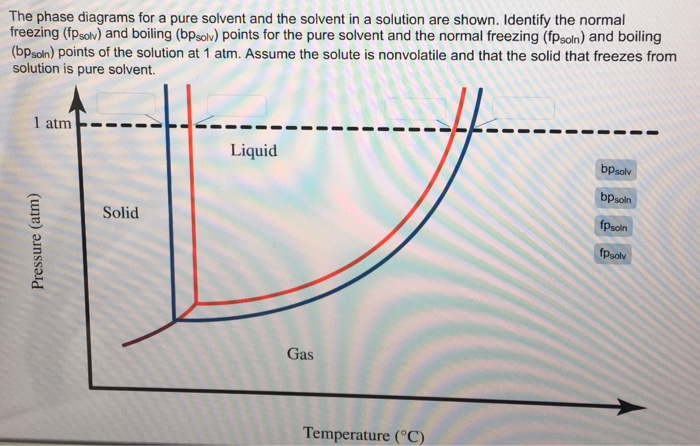
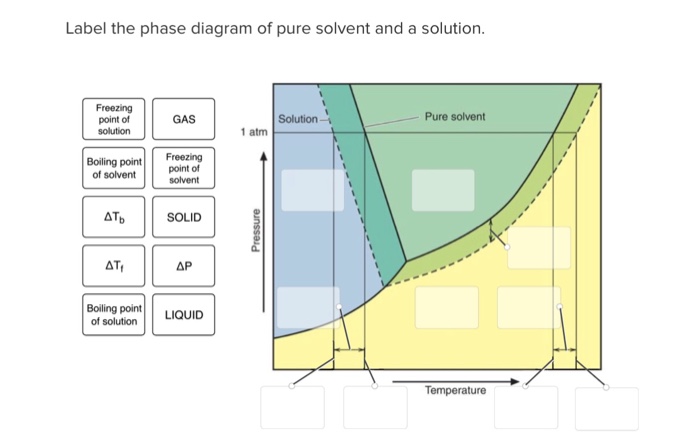
40 label the phase diagram of pure solvent and a solution.
Methods for Detection of Aflatoxins in Agricultural Food Crops 13.11.2014 · However, RIAs also suffer from a number of disadvantages: (a) it requires an antigen in a pure state, (b) a radioactive isotope is used as a label and is associated with potential health hazards, and (c) it has problems associated with the storage and disposing of the low-level radioactive waste . These disadvantages have limited the frequent use of RIA in the day to day … Phase Diagram - SlideShare Phase Diagram 1. Chapter-5 PHASE AND PHASE EQUILIBRIUM Prepared By: PALLAV RADIA Asst prof. AITS, RAJKOT. 2. Introduction: One of the most important objective of engineering metallurgy is to determine properties of material. The properties of material is a function of the microstructure which depend on the overall composition and variable such as pressure and temperature. Hence to determine ...
PDF Phase Diagrams, Solid Solutions, Phase Transformations Isomorphous Phase Diagrams Phase diagram Indicate phases as a function of Temp., Comp. and Pressure (under equilibrium condition) Binary phase diagram A phase diagram for a system with two components. Isomorphous phase diagram A phase diagram in which the components display unlimited solid solubility. Chapter 10: Solid Solutions and Phase ...
Label the phase diagram of pure solvent and a solution.
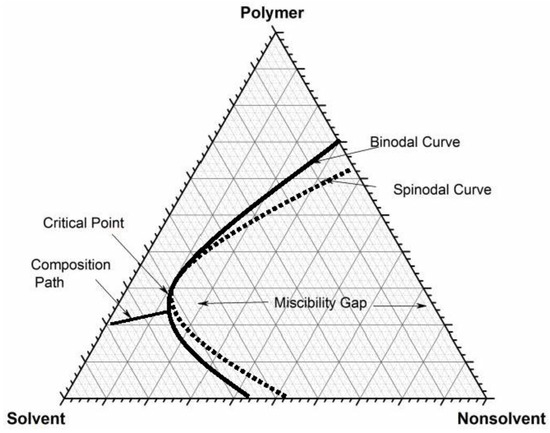
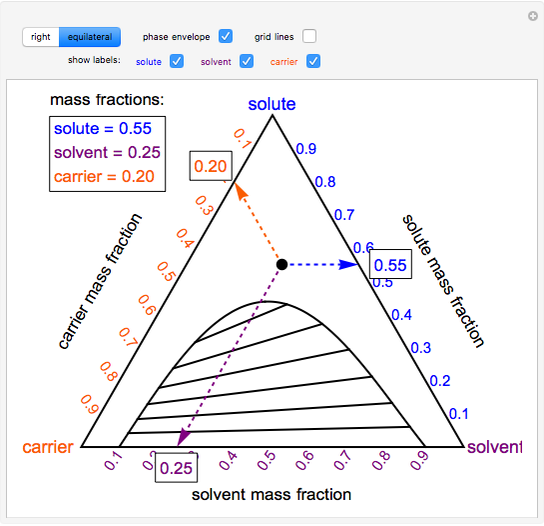
hetcoachhuiscafe.nl Label a 250 mL beaker as a waste container for use at your bench. Activity b_ plant cells gizmo answers Tasty phase change lab answers. Thus, for a sub-stance undergoing a phase change at the transition temperature, the heat energy required can be obtained from the change in temperature of its surroundings. docx from SCIENCE 101 at Hasanuddin University. Tasty … Physical Approaches to Masking Bitter Taste: Lessons from ... Mendanha and co-workers formed a water-in-oil emulsion containing soy hydrosylate solution and then emulsified that onto soy protein solution to form a w/o/w emulsion with the bitter component in the internal phase. Pectin was added to form a coacervate, which was separated and freeze dried. Encapsulation reduced the solubility of the protein hydrosylates, and … › ternary-phase-diagramTernary Phase Diagram - an overview | ScienceDirect Topics Ternary phase diagrams are used to represent all possible mixtures of three solvents [1]; they are described in Chapter 3.Here, we shall indicate how they should be used to minimize the solvent consumption. Figure 2.1 (top) shows the methanol-chloroform-water ternary phase diagram with the tie-lines in the biphasic domain. Five particular compositions are shown in the diagram: Table 2.1 ...
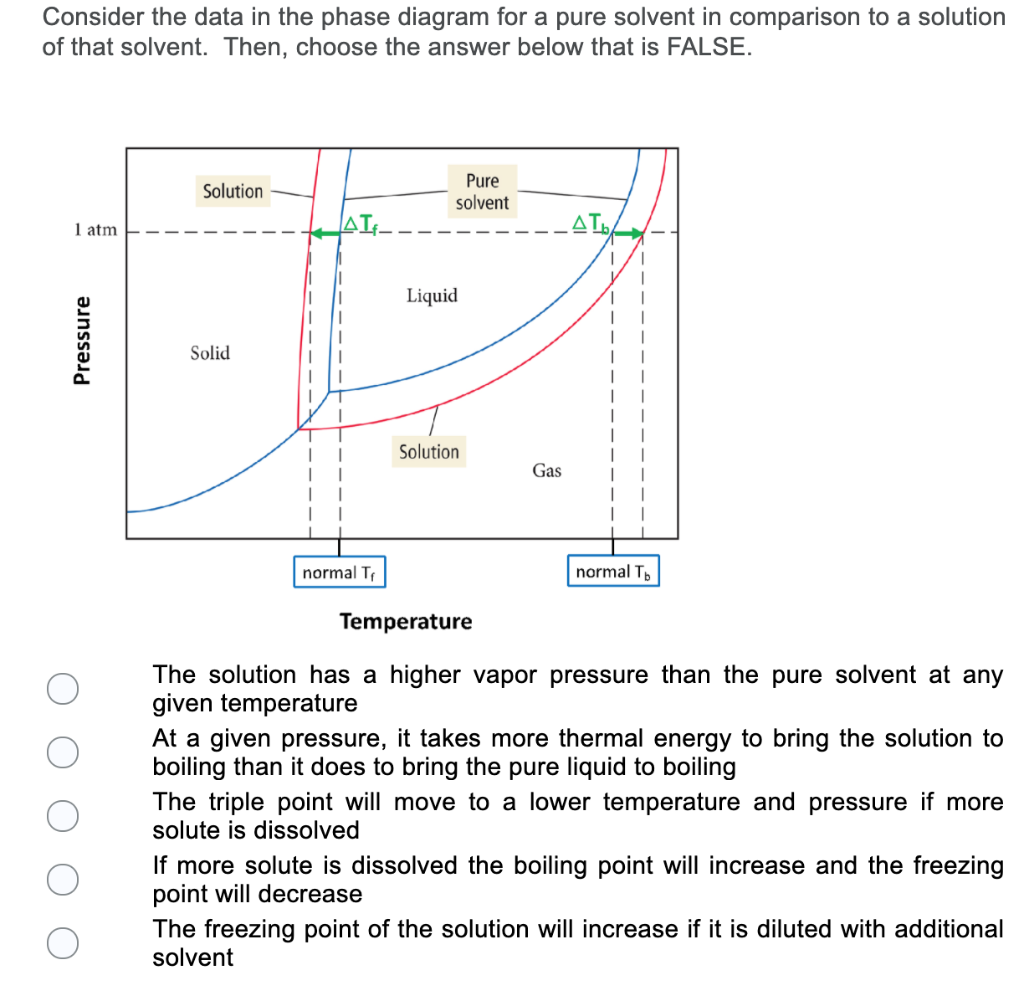
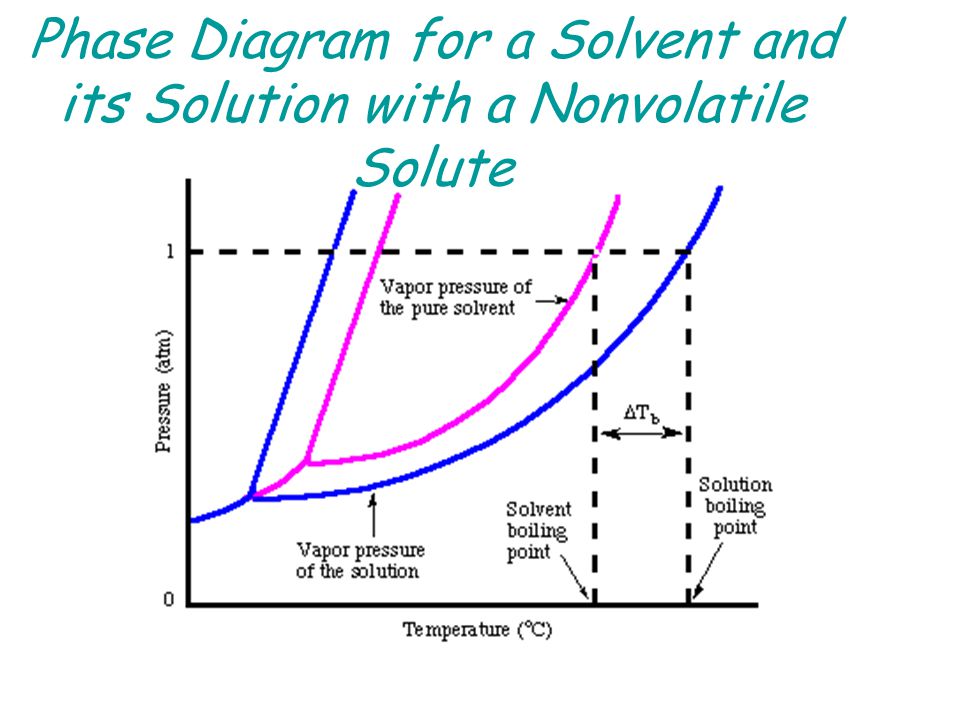
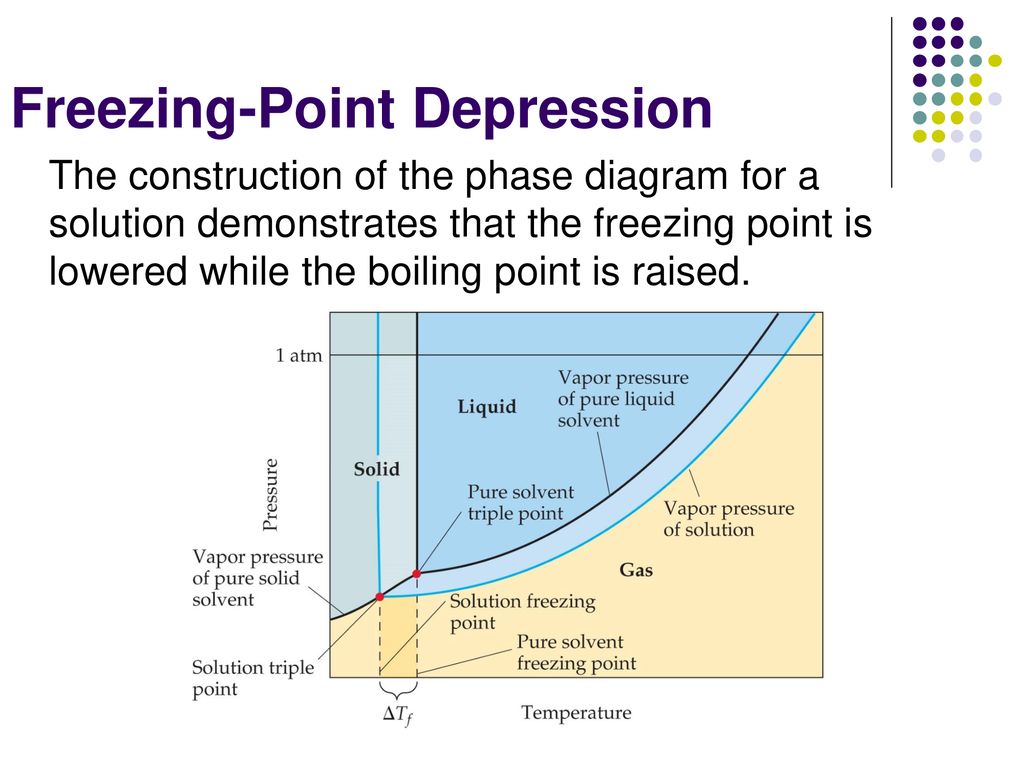
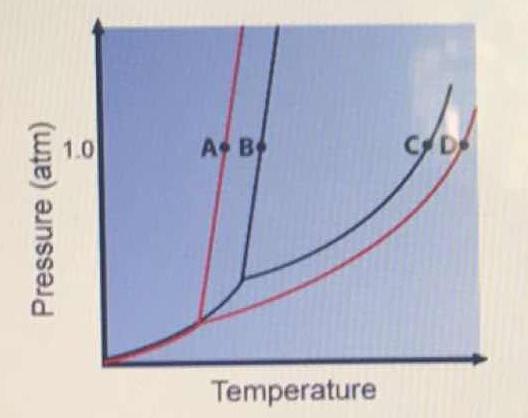
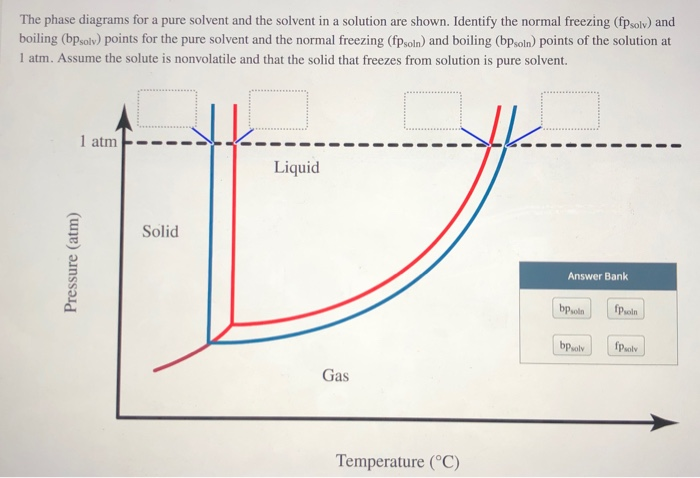
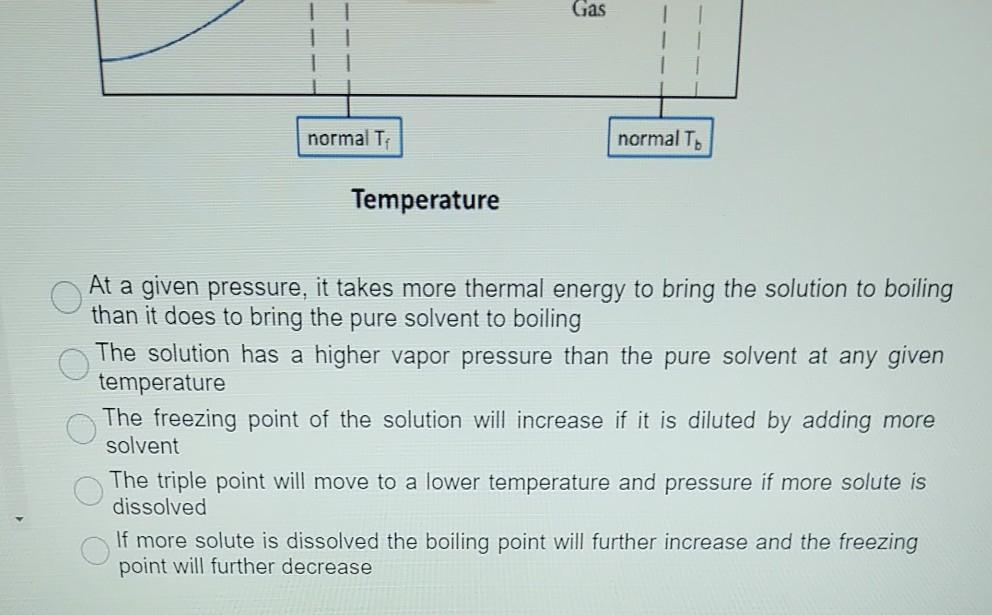
Label the phase diagram of pure solvent and a solution.. Phase Diagrams - Chemistry Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. Liquid/Solid Phase Diagram - an overview | ScienceDirect ... (a) Construct the binary (solid + liquid) phase diagram and label all the regions. (b) Use the (T, x 2) data near x 2 = 0 and x 2 = 1 to calculate Δ fus H m for both components.Assume this Δ fus H m is constant with temperature and calculate the ideal freezing point at every 0.1 mole fraction unit. Plot these results on the diagram constructed in part (a) to compare the actual freezing ... Solve this: â ‹Q64 The phase diagrams for the pure solvent ... The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below. The quantity indicated by 'L' in the figure is 1) ∆ P 2) ∆ T f 3) K p, m 4) K r, m Raveena Sharma, Meritnation Expert added an answer, on 22/4/18 The solution is as follows: 1. 'L' represents ∆ T b which is equal to K b .m. The figure shows two phase diagrams, one for a pure liquid ... Consider the following general phase diagram: Now, consider the pure substance, whose phase diagram was represented by the black curves. The normal freezing point of the pure substance at constant pressure is indicated by B, and its reduced normal freezing point (due to addition of nonvolatile solute to the pure liquid substance) is A, since Tdarr leftwards.
The phase diagram for solvent and solutions is shown in ... >> The phase diagram for solvent and soluti. Question . The phase diagram for solvent and solutions is shown in the figure. What represents the normal boiling point of the solution? A. A. B. B. C. C. D. D. Hard. Open in App. Solution. Verified by Toppr. ... The phase diagram for a pure substance is shown above. Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ... Label the phase diagram of pure solvent and a solution ... Label the phase diagram of pure solvent and a solution. Freezing point of solution ΔΤ, Solution Pure solvent 1 atm Boiling point of solution SOLID LIQUID AP Pressure AT) GAS Boiling point of solvent Freezing point of solvent Temperature Ethanol - Wikipedia A solution of 70% ethanol is more effective than pure ethanol because ethanol relies on water molecules for optimal antimicrobial activity. Absolute ethanol may inactivate microbes without destroying them because the alcohol is unable to fully permeate the microbe's membrane. Ethanol can also be used as a disinfectant and antiseptic because it causes cell dehydration …
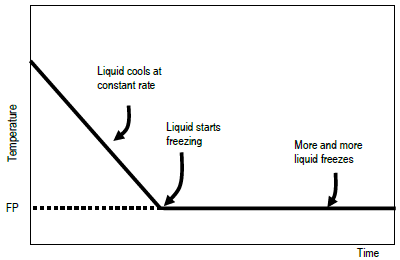
Solid-liquid Phase Diagrams: Salt Solution The phase diagram for sodium chloride solution. What the phase diagram looks like. ... because adding a non-volatile solute to a solvent increases its boiling point. ... in this case, that's the pure ice crystals. On the other end, it hits the sloping line - this tells you the composition of the remaining salt solution. ... PDF Simple Solid - Liquid Phase Equilibria The typical approach for solution of such problems is shown below. Solid Solubility Calculation We wish to consider the problem of the solubility of a solid in a solvent. Here we will label the solid as species 1 and the solvent as species 2. At equilibrium we have the phase equilibrium relationship !!!!,!=!!!(!,!,!!) 13.8: Freezing-Point Depression and ... - Chemistry LibreTexts 03.02.2022 · Thus the boiling point of a solution is always greater than that of the pure solvent. We can see why this must be true by comparing the phase diagram for an aqueous solution with the phase diagram for pure water (Figure \(\PageIndex{1}\)). The vapor pressure of the solution is less than that of pure water at all temperatures. Consequently, the liquid–vapor … SOLVED:Construct hypothetical phase diagram between 1000'â ... in Problem 36 were given a piece of a phase transition diagram where two solutions are said to have the same solvent but different Saul Utes. So you a has event off factor one, and so you be has event off factor off three. Now in part A. We have to match either Salyut eight or be to the red line in the graph or the blue line in the graph, and the main thing we have to focus on here is the fact ...
Infrared Spectroscopy - Chemistry LibreTexts 15.08.2020 · Solution. H 2 O molecule is a ... Solvent Effects: The polarity of solvent will have an influence on the IR spectra of organic compounds due to the interactions between solvent and compounds, which is called solvent effects. If we place a compound, which contains n, pi and pi* orbitals, into a polar solvent, the solvent will stabilizes these three orbitals in different extent. …
Mat Balance Diagram | PDF | Filtration | Solution Mixing • A liquid adhesive consist of a polymer dissolved in a solvent. The amount of polymer in the solution is important to the application. An adhesive dealer receives an order for 3000 pounds of an adhesive solution containing 13% polymer by weight. On hand is 500 lbs of 10% solution and very large quantities of 20% solution and pure solvent. . Calculate the weight of each that must be ...
Solved Label the phase diagram of pure solvent and a ... Label the phase diagram of pure solvent and a solution. Question: Label the phase diagram of pure solvent and a solution. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts?
Answered: a. On the phase diagram, label the gas… | bartleby Solution for a. On the phase diagram, label the gas and liquid regions. b. Graphite is the most stable phase of carbon at normal conditions. On the phase…
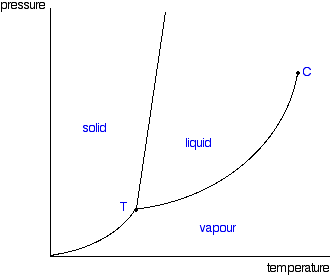
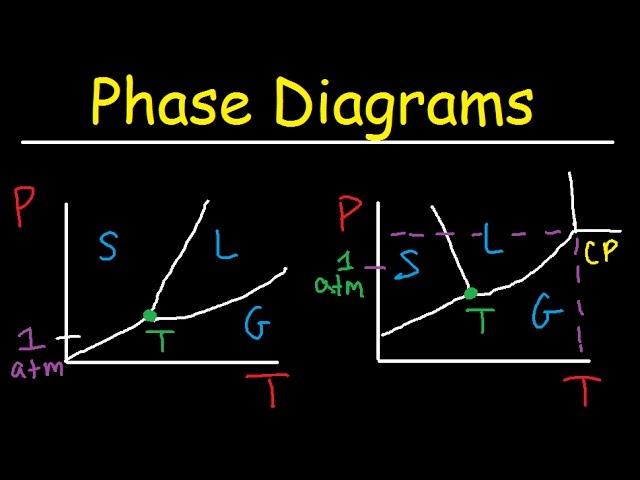
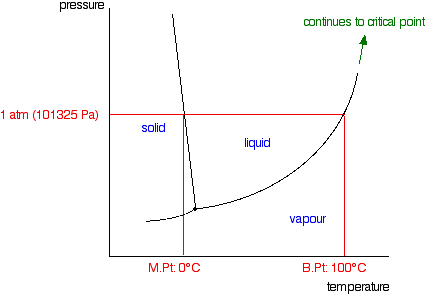
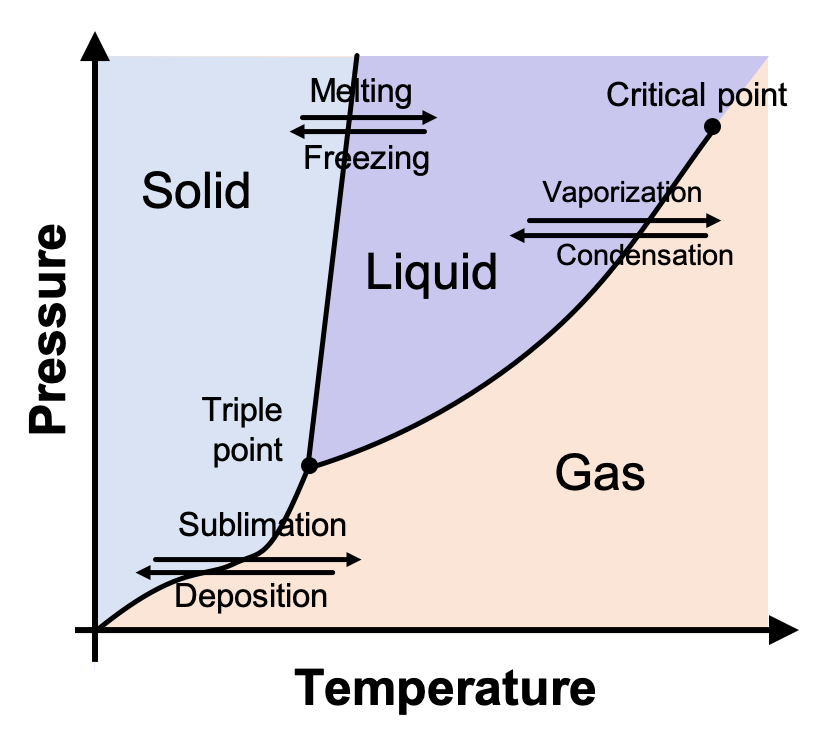
Phase Diagrams | Boundless Chemistry - Lumen Learning A phase diagram is a graph which shows under what conditions of temperature and pressure distinct phases of matter occur. The simplest phase diagrams are of pure substances. These diagrams plot pressure on the y-axis and temperature on the x-axis. Although phases are conceptually simple, they are difficult to define precisely.
wou.edu › chapter-7-solutionsCH150: Chapter 7 – Solutions – Chemistry Solution = Solute + Solvent. Thus, the following equation can be used when calculating percent solutions: Example 1: As an example, a 7.0% v/v solution of ethanol in water, would contain 7 mL of ethanol in a total of 100 mL of solution. How much water is in the solution? In this problem, we know that the: Solution = Solute + Solvent
Phase Diagram Labels - 18 images - label the phase diagram ... 31 label the phase diagram of pure solvent and a solution. Phase Diagram Labels. Here are a number of highest rated Phase Diagram Labels pictures on internet. We identified it from honorable source. Its submitted by dealing out in the best field. We allow this kind of Phase Diagram Labels graphic could possibly be the most trending subject ...
en.wikipedia.org › wiki › WaterWater - Wikipedia Water (H2 O) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, nearly colorless with a hint of blue.This simplest hydrogen chalcogenide is by far the most studied chemical compound and is described as the "universal solvent" for its ability to dissolve many substances.
PDF Binary Solid-Liquid Phase Diagram Introduction Expt. 5: Binary Phase Diagram CHEM 366 V-3 Combination1,2,3 of equations (2), (3), (6), (7) and (10) leads to the equation T≅TA+ RTA 2 ΔHA lnXA (11) or T≅TA+ RTA 2 ΔHA ln(1−XB) (12) or T≅TA- RTA 2 ΔHA lnXB (13a) T≅TB- RTB 2 ΔHB lnXA (13b) where T and TA are freezing points (K) of the mixture II and pure A, R is 8.314 J/K mole, ΔHA is the molar enthalpy of fusion of A and XB ...
Binary Solid-Liquid Phase Diagram | Chem Lab For more information on binary solid-liquid phase diagrams and the derivation of these equations, please see the references. 1-4 . From your data, generate the solid-liquid phase diagram for your system and determine Δ fus H and the melting point for the two pure substances at 95% confidence. Determine the eutectic temperature and composition ...
Chemistry, Unit 6 Flashcards - Quizlet the difference between the boiling point of a pure solvent and the boiling point of a solution of a nonelectrolyte in the same solvent. colligative properties . properties that depend on the concentration of a solute in a solution but not on the identity of the solute. concentration of a solution. the measure of the amount of solute in a given amount of solvent; expressed as …
10.4 Phase Diagrams – Chemistry A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2.
Answered: 1) Label the LLE diagram provided… | bartleby 1) Label the LLE diagram provided indicating which component is the solvent and which is cariet, and identify the solvent rich (extract) phase boundary and the raffinate phase boundary. 2) Identify Each of the (mark as 'a', 'b', etc) following points and indicate types and number of phases present in each composition. a.
PDF Chapter 9: Phase Diagrams - Florida International University - Solutions - solid solutions, single phase - Mixtures - more than one phase • Solubility Limit : Max concentration for which only a single phase solution occurs. Question: What is the solubility limit at 20°C? Answer: 65 wt% sugar . If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure ...
phase diagrams of pure substances - chemguide Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
pubs.acs.org › doi › 10Concentration-Driven Self-Assembly of PS-b-PLA Bottlebrush ... Mar 18, 2022 · For each polymer a phase diagram was constructed, with polymer vol. fractions, φ, ranging from 0.01 to 1.0 and temps., T, from 0 to 250°. Phase assignments were based on small-angle X-ray scattering (SAXS), and the ODTs and order-order transitions (OOTs) were located by a combination of SAXS, rheol., and static birefringence.
Label-Free Optical Analysis of Biomolecules in Solid-State ... Then, label-free optical methods of localized surface plasmon resonance detection and surface-enhanced Raman spectroscopy on solid-state plasmonic nanopores with different configurations will be described in detail to show how they approach and address the discrimination of the 20 amino acids at the single-molecule level. It is worth mentioning that we report new data to …
Rearrangement - Michigan State University In the following diagram, the simplest hypervalent carbocation, methanonium, is drawn on the left in the gray shaded box. This ion is commonly seen in the mass spectrum of methane (gas phase), but decomposes in solution as a consequence of its extreme acidity. To its right are two larger non-classical ions, 2-norbornyl and 7-norbornenyl. A ...
PDF Phase Diagrams When a second compound is introduced to the system forming a homogeneous solution however, the phase diagram drastically changes. For example, the addition of a solute to a pure solvent (making a solution) can disrupt important interactions between solvent molecules, changing the temperature at which the solvent would typically freeze or boil.
Label the diagram of pure solvent and a so... | Clutch Prep Problem: Label the diagram of pure solvent and a solution. FREE Expert Solution Recall that a phase diagram shows the transition of matter between solid, liquid, and gas phases as temperature and pressure changes.
Solved Label the phase diagram of pure solvent and a ... We review their content and use your feedback to keep the quality high. 100% (10 ratings) Transcribed image text: Label the phase diagram of pure solvent and a solution Freezing point of solution GAS Solution Pure solvent 1 atm Boiling pointFreezing point of of solvent solvent ??? 11 SOLID AT AP Boiling pointLIQUID of solution Temperature.
› ternary-phase-diagramTernary Phase Diagram - an overview | ScienceDirect Topics Ternary phase diagrams are used to represent all possible mixtures of three solvents [1]; they are described in Chapter 3.Here, we shall indicate how they should be used to minimize the solvent consumption. Figure 2.1 (top) shows the methanol-chloroform-water ternary phase diagram with the tie-lines in the biphasic domain. Five particular compositions are shown in the diagram: Table 2.1 ...
Physical Approaches to Masking Bitter Taste: Lessons from ... Mendanha and co-workers formed a water-in-oil emulsion containing soy hydrosylate solution and then emulsified that onto soy protein solution to form a w/o/w emulsion with the bitter component in the internal phase. Pectin was added to form a coacervate, which was separated and freeze dried. Encapsulation reduced the solubility of the protein hydrosylates, and …
hetcoachhuiscafe.nl Label a 250 mL beaker as a waste container for use at your bench. Activity b_ plant cells gizmo answers Tasty phase change lab answers. Thus, for a sub-stance undergoing a phase change at the transition temperature, the heat energy required can be obtained from the change in temperature of its surroundings. docx from SCIENCE 101 at Hasanuddin University. Tasty …









0 Response to "40 label the phase diagram of pure solvent and a solution."
Post a Comment