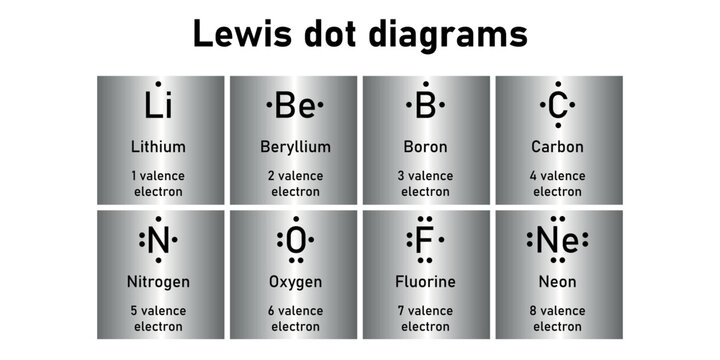
44 lewis dot diagram boron
Lewis dot structure of boron? - Answers The Lewis dot diagram for Boron has three dots. What is the Lewis dot structure for sodium borohydride? This is an ionic compound. Sodium is positively charged and is paired with the negatively ... Solid - Wikipedia The bulk of the elements in the periodic table, those to the left of a diagonal line drawn from boron to polonium, are metals. Mixtures of two or more elements in which the major component is a metal are known as alloys. People have been using metals for a variety of purposes since prehistoric times.
Lewis Dot Structure for Boron||How do you draw the Lewis Dot structure ... Lewis Dot Structure for Boron||How do you draw the Lewis Dot structure for Boron atom?||Lewis Symbol for Boron
Lewis dot diagram boron
Lewis Electron- Dot Structure of boron fluoride BF molecule - #41 - Blogger Step 1: Connect the atoms with single bonds. Fig. 1 : Connect the atoms of the BF molecule with single bonds. Step 2: Calculate the # of electrons in π bonds (multiple bonds) using Lewis structure formula (1): Where n in this case is 2 since BF consists of two atoms. Where V = (3 + 7) = 10 , V is the number of valence electrons of the ion. Lewis Electron Dot Diagrams – Introductory Chemistry – 1st ... Because the side is not important, the Lewis electron dot diagram could also be drawn as follows: The electron dot diagram for helium, with two valence electrons, is as follows: By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1 s subshell; this is the common convention we ... How to draw Bohr diagram for Oxygen(O) atom - Topblogtenz Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Oxygen, we got to know, it has 6 valence electrons. So, just represent these 6 valence electrons around the Oxygen atom as a dot.
Lewis dot diagram boron. Lewis Dot Diagram For Boron An electron 13, Electron dot diagram for boron. The unpaired electron is usually placed in the Lewis Dot Structure so The problem with this structure is that boron has an incomplete octet;. Structure, properties, spectra, suppliers and links for: Boron nitride. Boron has 5 electrons. 3 are in the valence shell. Boron Lewis Dot Structure: Drawing, Several Compounds and Detailed ... The Lewis dot structure of Boron trichlortde enhances the idea about sharing electrons. Boron has three valance electrons and three chlorine atoms have (3*7= 21) valance electrons. Therefore, total amount of valance electrons take place in the formation of BCl3 is 24. BF3 Lewis Structure, Molecular Geometry, and Hybridization Nov 30, 2022 · Finding out Lewis Structure of BF3. The periodic table helps you to study various elements that include atomic number, valency, etc. To learn about any Lewis dot structure of boron trifluoride BF3, you need to compute mainly four important things. The total number of valence electrons. Required number of electrons to complete octet. Boron trifluoride (BF3) lewis dot structure, molecular geometry, polar ... Boron trifluoride (BF3) lewis structure comprises of three B-F bonds, with boron in a central position and all three fluorine as outer atoms in the lewis diagram. The lewis dot structure of BF3 contains a total of 3 bond pairs and 9 lone pairs. The drawing of the Lewis structure of BF 3 is very easy and simple. Let's see how to do it.
9.9: Exceptions to the Octet Rule - Chemistry LibreTexts Jun 24, 2021 · However, boron has an electronegativity that is very similar to hydrogen, meaning there is likely very little ionic character in the hydrogen to boron bonds, and as such this Lewis structure, though it does not fulfill the octet rule, is likely the best structure possible for depicting BH 3 with Lewis theory. Boron triiodide (BI3) lewis dot structure, molecular geometry, polar or ... The lesser the formal charge on atoms, the better is the stability of the lewis diagram. To calculate the formal charge on an atom. Use the formula given below- ⇒ Formal charge = (valence electrons - nonbonding electrons - 1/2 bonding electrons) Let's count the formal charge for the 4th step structure. For iodine atom 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry - Lumen Learning Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Exercises 1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. 2. How to Draw the Lewis Dot Structure for BN: Boron nitride How to Draw the Lewis Dot Structure for BN: Boron nitride 12,854 views Dec 10, 2018 A step-by-step explanation of how to draw the BN Lewis Dot Structure (Boron nitride). ...more ...more...
Lewis Dot Structure for Boron Atom (B) - YouTube Lewis Dot Structure for Boron Atom (B) 35,782 views Sep 13, 2013 A step-by-step explanation of how to draw the Lewis dot structure for B (Boron). I show you where Boron is on the periodic... Lewis Symbols and Structures – Chemistry For example, in the Lewis structures of beryllium dihydride, BeH 2, and boron trifluoride, BF 3, the beryllium and boron atoms each have only four and six electrons, respectively. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3 , satisfying the octet rule, but experimental evidence ... Lewis Electron Dot Diagrams - GitHub Pages Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. How to draw the B3+ Lewis Dot Structure. - YouTube For the B3+ structure use the periodic table to find the total number of valence electrons for B. Once we know how many valence electrons there are in Boron ...
which lewis electron dot diagram represents a boron atom in the ground ... Answer : The Lewis-dot structure of boron atom is shown below. Explanation : Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. In the Lewis-dot structure the valance electrons are shown by 'dot'.
BF3 Lewis Structure: How to Draw the Lewis Structure for BF3 In the Lewis structure of BF3 structure there are a total of 24 valence electrons. BF3 is also called Boron trifluoride. ----- Steps to Write Lewis Structure for compounds like BF3...
CH3Br Lewis Structure, Geometry, Hybridization, and Polarity These are also referred to as electron dot structures as they depict the lone pair of electrons present around each atom of a molecule in the form of dots. ... is the stable Lewis structure. Related topics you must read. SiO2 Lewis Structure. N2F2 Lewis Structure. ... 11 Uses of Boron — Commercial, Biological, and Miscellaneous . August 16, 2022.
Lewis Electron Dot Structures - Detailed Explanation with ... - BYJUS Lewis structures, also known as electron-dot or electron-dot diagrams, are diagrams showing the bonding between a molecule's atoms and the lone pairs of electrons that may occur in the molecule. What is the Lewis structure of ammonia? Ammonia has the molecular formula NH 3. It is extremely water-soluble because it is a polar material.
How to Draw the Lewis Structure for BH3 - YouTube A step-by-step explanation of how to draw the BH3 Lewis Dot Structure (Boron Trihydride).There are only 6 valence electrons in the Lewis structure for BH3.Th... AboutPressCopyrightContact...
CN- lewis structure, molecular orbital diagram, bond order ... There are 2 lone pairs present, one on each atom in CN – Lewis dot structure. A -1 formal charge accounts for the one extra valence electron present in the CN – Lewis structure. The molecular orbital diagram of CN – shows the placement of electrons in molecular orbitals after C-N bond formation. The bond order of CN – is 3 while that of ...
How to Draw the Lewis Dot Structure for BrF3: Boron trifluoride A step-by-step explanation of how to draw the BrF3 Lewis Dot Structure (Boron trifluoride ).For the BrF3 structure use the periodic table to find the total n... AboutPressCopyrightContact...
Lewis Dot Structure: An Overview | Borates Today Here are some steps on how to draw lewis dot diagram of a moleculeto follow while drawing a lewis dot structure of any molecule: Step 1: Calculate the Total Number of Valence Electrons Sum up the valence electrons in the molecule from all the atoms. Step 2: Determine how many Electrons are required to make the Atoms "Happy."
How to draw Bohr diagram for Oxygen(O) atom - Topblogtenz Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Oxygen, we got to know, it has 6 valence electrons. So, just represent these 6 valence electrons around the Oxygen atom as a dot.
Lewis Electron Dot Diagrams – Introductory Chemistry – 1st ... Because the side is not important, the Lewis electron dot diagram could also be drawn as follows: The electron dot diagram for helium, with two valence electrons, is as follows: By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1 s subshell; this is the common convention we ...
Lewis Electron- Dot Structure of boron fluoride BF molecule - #41 - Blogger Step 1: Connect the atoms with single bonds. Fig. 1 : Connect the atoms of the BF molecule with single bonds. Step 2: Calculate the # of electrons in π bonds (multiple bonds) using Lewis structure formula (1): Where n in this case is 2 since BF consists of two atoms. Where V = (3 + 7) = 10 , V is the number of valence electrons of the ion.

0 Response to "44 lewis dot diagram boron"
Post a Comment