42 square pyramidal mo diagram
en.wikipedia.org › wiki › Metal_ions_in_aqueous_solutionMetal ions in aqueous solution - Wikipedia Another aqua species in which there is a metal-metal bond is the molybdenum(II) species formulated as [(H 2 O) 4 Mo≣Mo(H 2 O) 4] 4+. Each molybdenum is surrounded by four water molecules in a square-planar arrangement, in a structure similar to that of the known structure of the chloro complex [Mo 2 Cl 8] 4−. A transcriptomic and epigenomic cell atlas of the mouse WebOct 6, 2021 · However, recent anatomical studies have identified a population of pyramidal cells located between L3 and L5, with hallmarks of L4 neurons including thalamic input and outputs to L4 and L2/3 (ref. 4).
Currently Unstable: Daily Ups and Downs in E-I Balance Webpyramidal neurons in slices of mouse visual cortex. Slices were harvested at either zeitgeber time 0 (ZT0; lights on) or ZT12 (lights off). While mini amplitudes, a measure of synaptic strength, were equivalent at both time points, mEPSC frequency was higher at ZT0 than ZT12. Surprisingly, changes in mIPSC fre-quency were inverted, resulting in
Square pyramidal mo diagram
Molecular orbital energy levels in square-pyramidal Co(CN)3−5 ion An SCCC molecular orbital calculation was performed for low spin square-pyramidal Co (CN) 3−5. The results show that the ordering of the d levels xy < xz, yz < z2 ⪡ x2 - y2, which is in unexpected from the point of view of crystal field theory, is due to the fact that the metal lies above the equatorial plane of the ligands. Previous article Transition Metal d-Orbital Splitting Diagrams: An Updated Educational ... The presentation of d-orbital splitting diagrams for square planar transition metal complexes in textbooks and educational materials is often inconsistent and therefore confusing for students. Here we provide a concise summary of the key features of orbital splitting diagrams for square planar complexes, which we propose may be used as an updated reference in chemical education. Molecular Structure & Bonding - Michigan State University WebFor purposes of discussion we shall consider three other configurations for CH 4, square-planar, square-pyramidal and triangular-pyramidal. ... The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher ...
Square pyramidal mo diagram. ICl3 Lewis Structure, Molecular Geometry, Hybridization, and … WebDec 7, 2022 · Square pyramidal: AX4E2: 4: 2: Octahedral: Square planar: ... Hybridization, and MO Diagram. Next Article OF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Leave a Reply Cancel reply. Your email address will not be published. Required fields are marked * Comment. Building Molecular Orbitals for a Square Pyramidal Oxorhenium(V ... -build a MO diagram for a square pyramidal complex. -include the effect of ligands with sigma-donating, pi-donating and/or pi-accepting character. Related activities Oxorhenium (V) Methyl, Benzyl, and Phenyl Complexes: New Mechanism for Carbonyl Insertion Implementation Notes Students should work in small groups of 3-4. Time Required d-Metal Complexes - uml.edu This distortion to square planar complexes is especially prevalent for d 8 configurations and elements in the 4 th and 5 th periods such as: Rh (I), Ir (I), Pt (II), Pd (III), and Au (III). Nickel (II) four-coordinate complexes are usually tetrahedral unless there is a very strong ligand fields such as in [Ni (CN) 4] 2-, which is square planar. Microsoft takes the gloves off as it battles Sony for its Activision ... WebOct 12, 2022 · Microsoft pleaded for its deal on the day of the Phase 2 decision last month, but now the gloves are well and truly off. Microsoft describes the CMA’s concerns as “misplaced” and says that ...
Point Group C4v - an overview | ScienceDirect Topics "True" oxyfluoride compounds usually have a relatively high F/O ratio and crystallize in the following types of structures: K 3 MoO 3 F 3, Bi 2 TiO 4 F 2, Na 5 W 3 O 9 F 5, Pb 5 W 3 O 9 F 10 and Mg 3 B 7 O 13 F. The second group is the group of oxyfluorides that are derived from ferroelectric oxides by means of fluorine-oxygen substitution. Square pyramidal molecular geometry - Wikipedia In molecular geometry, square pyramidal geometry describes the shape of certain compounds with the formula ML5 where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. The point group symmetry involved is of type C 4v. › 2022/10/12 › 23400986Microsoft takes the gloves off as it battles Sony for its ... Oct 12, 2022 · Microsoft pleaded for its deal on the day of the Phase 2 decision last month, but now the gloves are well and truly off. Microsoft describes the CMA’s concerns as “misplaced” and says that ... CHEM 123 Sapling Learning Chapter 11 Flashcards | Quizlet WebIn square pyramidal geometry, four bonding electron groups form the square plane around the central atom, whereas the fifth bonding group lies above the plane to form the top of the pyramid. ... For each of these molecules, identify the proper MO diagram and the number of valence electrons. The 1𝑠 orbital is not shown. Identify the MO ...
d-orbitals and ligand geometries - Beloit College square planar tetrahedral square pyramidal linear trigonal bipyramidal cubic dodecahedral square prism square antiprism trigonal prism trigonal antiprism. Which d orbitals overlap with the ligands? orbitals off 3d z 2 3d xz 3d yz 3d x 2-y 2 3d xy. Architecture of the medieval cathedrals of England - Wikipedia WebThe medieval cathedrals of England, which date from between approximately 1040 and 1540, are a group of twenty-six buildings that constitute a major aspect of the country's artistic heritage and are among the most significant material symbols of Christianity.Though diverse in style, they are united by a common function. As cathedrals, each of these … PDF D-orbital splitting diagrams - University of California, Berkeley D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. Trigonal bipyramidal 4. Square pyramidal d z2x2-y d xy d yzxz 5. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d ... PDF MO class activity - VIPEr b. Redraw your MO diagram for orbitals of metal d character in a square pyramidal complex. Label the orbitals. 3. Now consider the oxorhenium(V) complex synthesized by Elon Ison's group in Organometallics 2015, 34, 3152-3158. This complex is reported to have a "distorted" square pyramidal geometry.
chemistry 1a Chapter 10 Flashcards | Quizlet the result is called a square pyramidal shape. The bond angles between axial and equatorial positions is < 90°. If 6 electron group has 2 lone pair the result is called a square planar shape. The bond angles between equatorial positions is 90°.
PDF MO Diagrams for More Complex Molecules - University of California, Irvine MO Theory • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
quizlet.com › 552869460 › chem-123-sapling-learningCHEM 123 Sapling Learning Chapter 11 Flashcards | Quizlet M O diagram A has the following orbitals from lowest energy to highest: sigma 2 s, antibonding sigma 2 s, pi 2 p, sigma 2 p, antibonding pi 2 p, and antibonding sigma 2 p. For each of these molecules, identify the proper MO diagram and the number of valence electrons. The 1𝑠 orbital is not shown. Identify the MO diagram for B2. B2 valence e−:
www2.chemistry.msu.edu › faculty › reuschMolecular Structure & Bonding - Michigan State University The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond.
Molecular orbitals square pyramidal - Big Chemical Encyclopedia Molecular orbital energy diagram for an AB5 molecule with the square pyramidal geometry. (No s orbital interaction.)... The VSEPR approach attributes this geometry to the electrostatic repulsion due to the sixth pair of electrons sticking out of the base of the square pyramid.
Solved 3) Draw a complete MO diagram for a square pyramidal - Chegg 3) Draw a complete MO diagram for a square pyramidal [Nicls] molecule (C4v) assuming the valence orbitals of Ni are the 3d and 4s orbitals. The SALCs for the o donors are 2an, bı, e, while the a SALCs are 2a1, a2, 2bı, b2, 2e. Show the splitting of the d manifold clearly. Identify one possible charge transfer band. This problem has been solved!
(PDF) Inorganic Chemistry Housecroft - Academia.edu WebTITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants, reductants, acids, bases, metal ions, etc. Titration is based on a reaction between the analyte (unknown sample) and the regent of …
› articles › s41586/021/03500-8A transcriptomic and epigenomic cell atlas of the mouse ... Oct 06, 2021 · However, recent anatomical studies have identified a population of pyramidal cells located between L3 and L5, with hallmarks of L4 neurons including thalamic input and outputs to L4 and L2/3 (ref. 4).
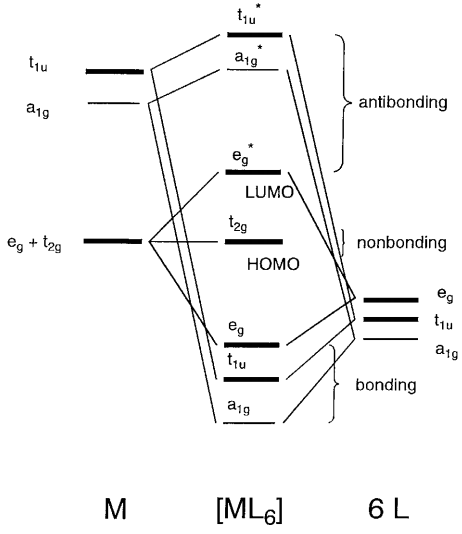
PDF Coordination Chemistry II: Jahn-Teller, Square Planar Complexes ... Jahn-Teller, Square Planar Complexes, Orbital Overlap Method, and Electron Counting Chapter 10 and Section 13.3 Monday, November 30, 2015. Jahn-Teller Distortions Jahn-Teller Theorem: electron configurations with unequal occupancy ... σ‐ML6Octahedral MO Diagram 1eg 2eg 1a1g 2a1g 1t1u 2t1u t2g
Metal ions in aqueous solution - Wikipedia WebA metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [M(H 2 O) n] z+.The solvation number, n, determined by a variety of experimental methods is 4 for Li + and Be 2+ and 6 for most elements in periods 3 and 4 of the periodic table. Lanthanide and actinide aqua ions have higher solvation numbers (often 8 to 9), …
Square Pyramidal - Chemistry LibreTexts Square Planar T-shaped AX 5 E NOTES: This molecule is made up of 6 equally spaced sp 3 d 2 hybrid orbitals arranged at 90 o angles. The shape of the orbitals is octahedral. One orbital contains a lone pair of electrons so the remaining five atoms connected to the central atom gives the molecule a square pyramidal shape.
quizlet.com › 538030724 › module-two-chem-101Module Two Chem 101 Problems Flashcards | Quizlet Study with Quizlet and memorize flashcards containing terms like Carbon dioxide is a _____ compound composed two types of _____ atoms. A. Molecular, metal B. ionic, metalloid C. molecular, nonmetal D. ionic, metal, Classify the following compounds as ionic or covalent: KCl, CrCl₃, Cl₂O. A. ionic, covalent, covalent B. ionic, ionic, covalent C. covalent, covalent, ionic D. ionic, covalent ...
› 34602222 › Inorganic_ChemistryInorganic Chemistry Housecroft - Academia.edu TITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants, reductants, acids, bases, metal ions, etc. Titration is based on a reaction between the analyte (unknown sample) and the regent of known concentration and reaction stoichiometry.
Module Two Chem 101 Problems Flashcards | Quizlet Weboctahedral / square pyramidal. Draw the Lewis structure of water (H₂O) and then determine its electron domain and molecular geometries. image 23 ... Use the MO diagram (below) to calculate the bond order for I₂. 1. The molecular orbital energy diagram for N₂ is shown below. Based on this diagram, is the molecule paramagnetic or diamagnetic?
BrF5 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Step 1: Find the total number of valence electrons one molecule of BrF5 has: It is 42 as 7 is coming from each of the fluorine and bromine atoms. Step 2: Find how many more valence electrons are required by one molecule of BrF5: It is 6 as one valence electron is required by each participating atom.
Molecular Structure & Bonding - Michigan State University WebFor purposes of discussion we shall consider three other configurations for CH 4, square-planar, square-pyramidal and triangular-pyramidal. ... The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher ...
Transition Metal d-Orbital Splitting Diagrams: An Updated Educational ... The presentation of d-orbital splitting diagrams for square planar transition metal complexes in textbooks and educational materials is often inconsistent and therefore confusing for students. Here we provide a concise summary of the key features of orbital splitting diagrams for square planar complexes, which we propose may be used as an updated reference in chemical education.
Molecular orbital energy levels in square-pyramidal Co(CN)3−5 ion An SCCC molecular orbital calculation was performed for low spin square-pyramidal Co (CN) 3−5. The results show that the ordering of the d levels xy < xz, yz < z2 ⪡ x2 - y2, which is in unexpected from the point of view of crystal field theory, is due to the fact that the metal lies above the equatorial plane of the ligands. Previous article

0 Response to "42 square pyramidal mo diagram"
Post a Comment