42 orbital diagram for hydrogen
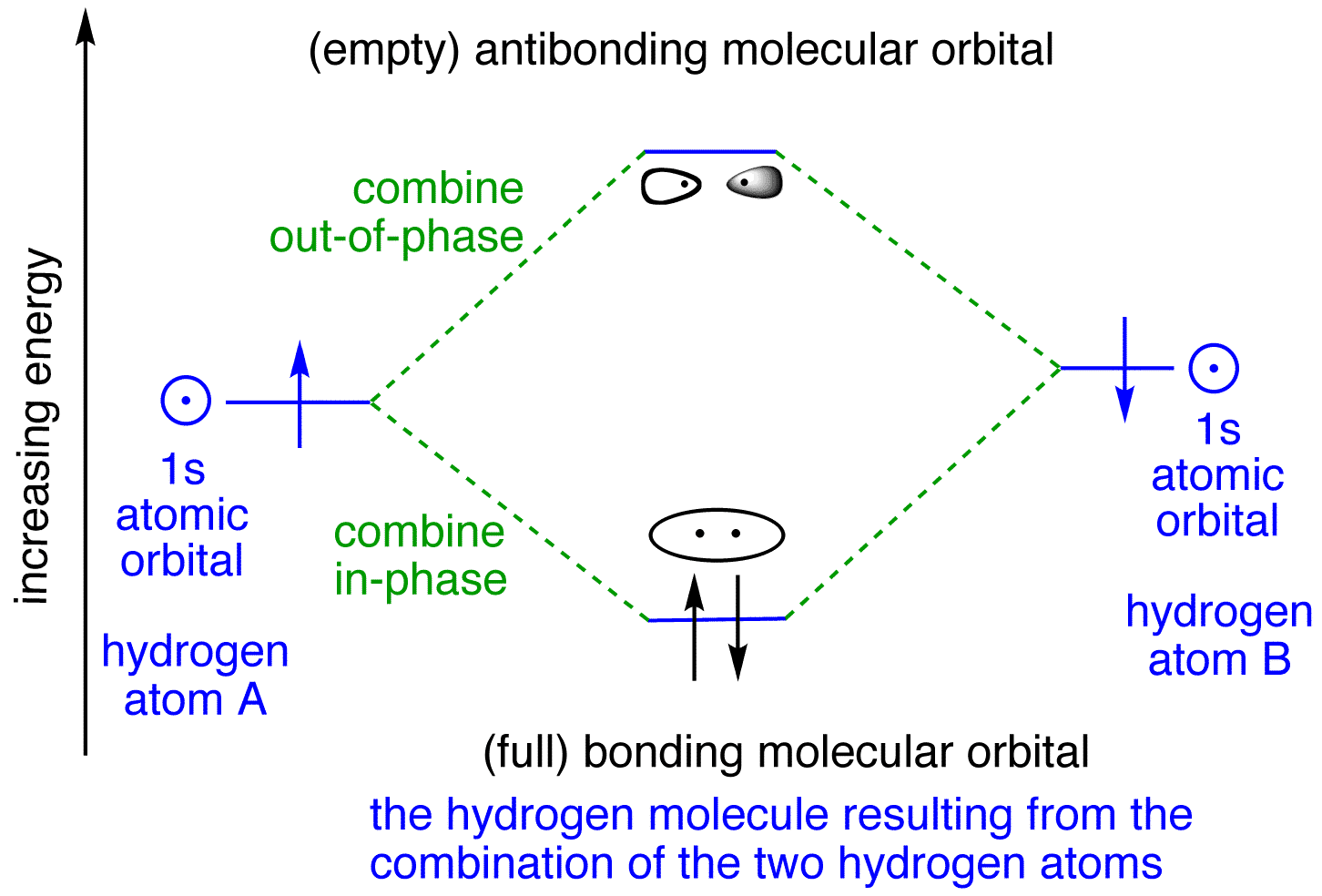
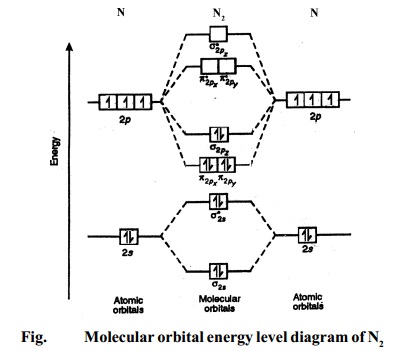
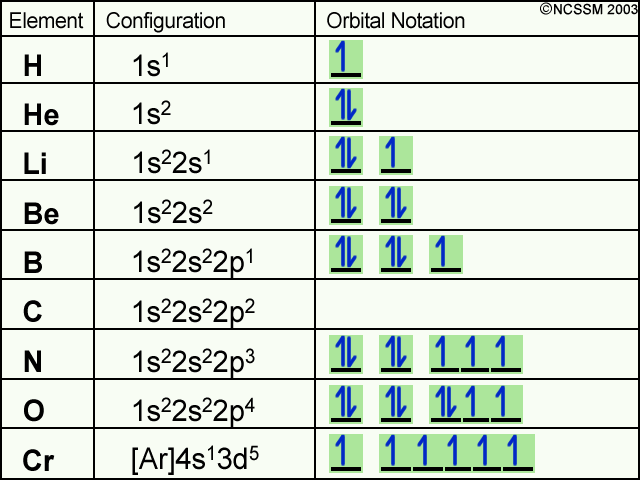
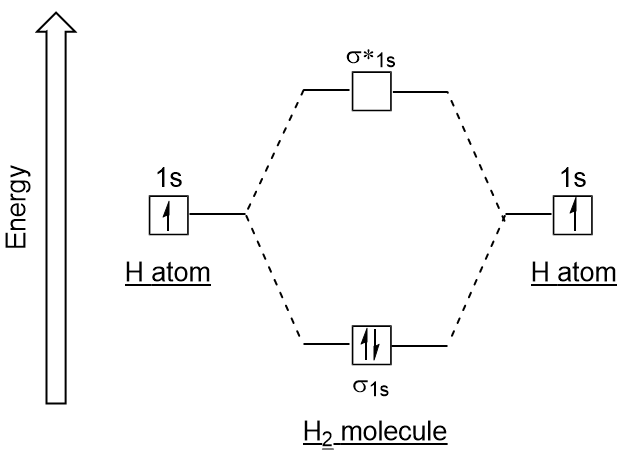
Nitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen Electron configuration of nitrogen in the excited state. Atoms can jump from one orbital to another in an excited state. This is called quantum jump. The ground-state electron configuration of nitrogen is 1s 2 2s 2 2p 3. We already know that the p-subshell has three orbitals. Bonding in Hydrogen - University of Illinois Urbana-Champaign In H2, we have 2 hydrogen atoms, each with a 1s orbital. These orbitals are pointing at each other along the z axis, so they will make sigma orbitals. We can make molecular orbitals by combining these 2 atomic orbital to obtain 2 molecular orbitals. One orbital comes from addition, {H11s + H21s}, and the other comes from subtraction, {H11s - H21s}.
Periodic Table - Ptable Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups.
Orbital diagram for hydrogen
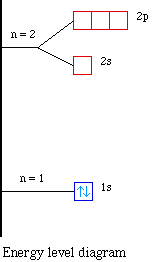
Why do energy level diagrams differ for hydrogen and You can actually use these energy level diagrams for helium, which (normally) has 2 electrons. What matters is that hydrogen and helium only have one orbital, which can very easily be … 2.5.5: Molecular Orbital Diagrams - Chemistry LibreTexts Molecular Orbital Diagrams This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals. Atomic and Molecular Hydrogen - University of Illinois Urbana-Champaign Below you can see an orbital energy diagram showing the ground state hydrogen atom on the left. When hydrogen absorbs a quantity of energy exactly equal to E 1, the electron goes from the orbital in the first shell (n = 1) to an orbital in the second shell (n = 2). This hydrogen molecule is in an excited state.
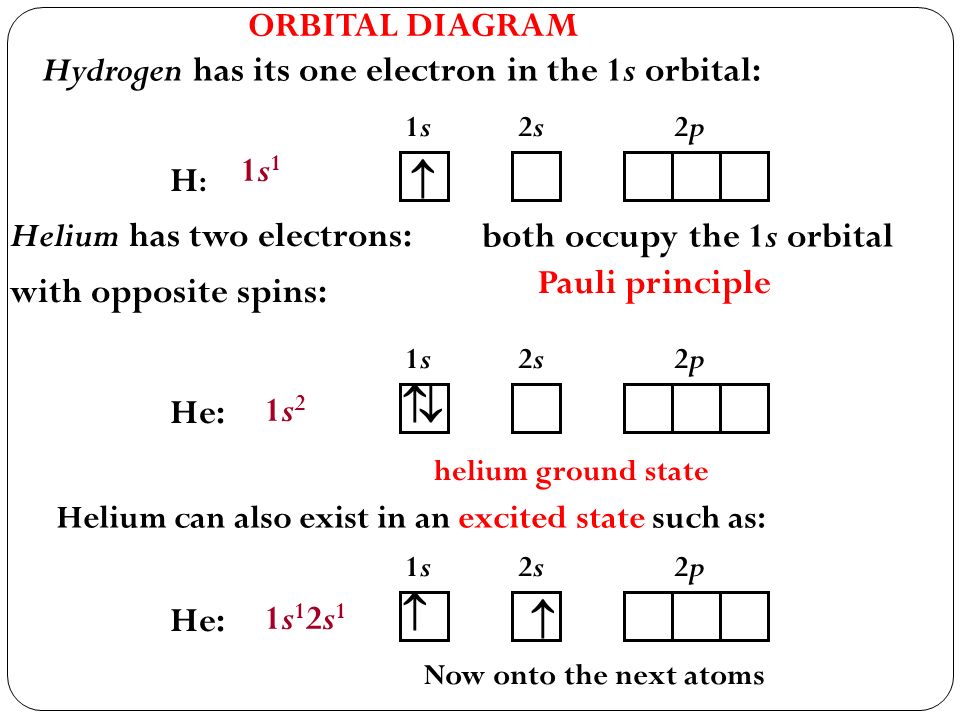
Orbital diagram for hydrogen. Atomic orbital - Wikipedia 3D views of some hydrogen-like atomic orbitals showing probability density and phase ( g orbitals and higher are not shown) Atomic orbitals can be the hydrogen-like "orbitals" which are exact solutions to the Schrödinger equation for a hydrogen-like "atom" (i.e., atom with one electron). Molecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. Orbital Diagrams Chemistry Tutorial - AUS-e-TUTE An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s. Hydrogen atom - Wikipedia A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic …
Molecular Orbitals - Introductory Chemistry - 1st Canadian Edition Similar to atomic orbitals, we can write electron configuration energy diagrams for molecular orbitals (Figure 9.20 "Hydrogen molecular orbital electron configuration energy diagram"). Notice that the atomic orbitals of each atom are written on either side, and the newly formed molecular orbitals are written in the centre of the diagram. Orbital structure of hydrogen atom, principal quantum number n, number ... The first quantum number n, describes the electron shell, or energy level, of an atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. Orbital filling diagrams | The Cavalcade o' Chemistry The orbital filling diagram for hydrogen As we've seen before, the electron configuration for hydrogen is: 1s¹ Let's draw the orbital filling diagram for hydrogen: Let's go through each of the elements of this picture so we don't get confused: The vertical arrow on the left with the word "Energy" next to it will be in all orbital filling diagrams. Oxygen Orbital diagram, Electron configuration, and ... - Topblogtenz Orbital diagram:- A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom, it shows the electrons in the form of arrows, also, indicates the spin of electrons. Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals.
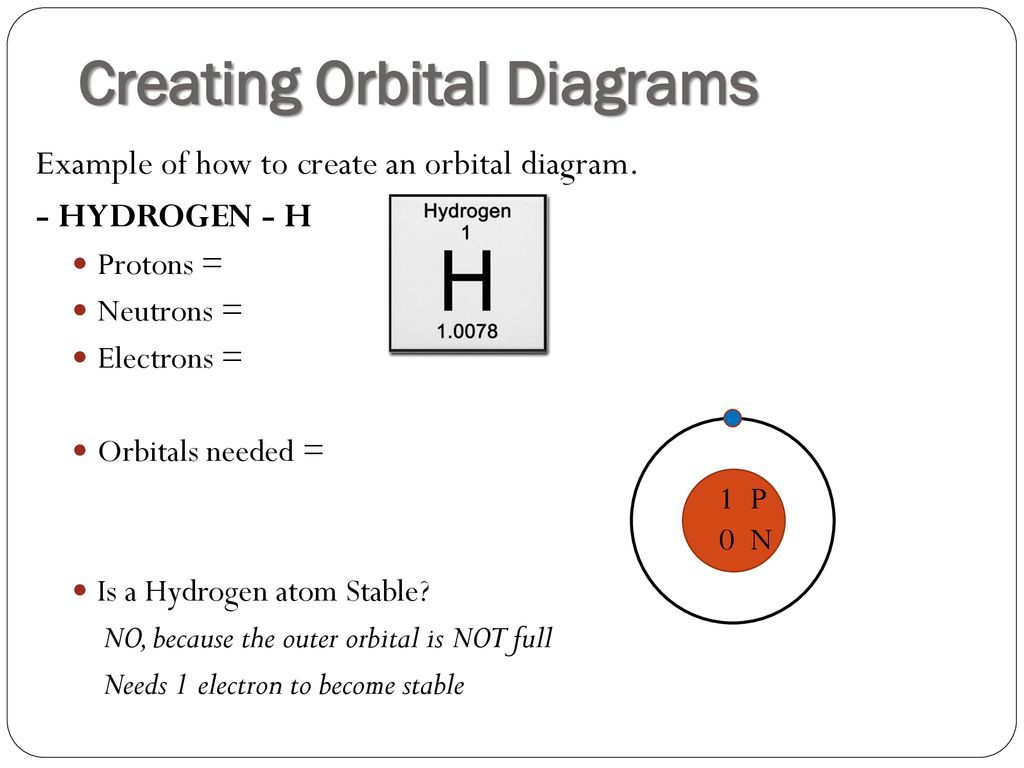
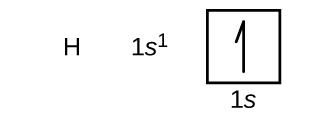
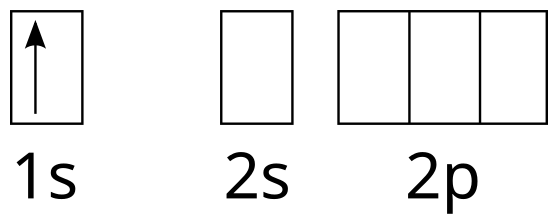
How to Write the Atomic Orbital Diagram for Hydrogen (H) To write the orbital diagram for the Hydrogen (H) first we need to write the electron configuration for just H. To do that we need to find the number of electrons for the H atom (there are 1... Electronic Orbitals - Chemistry LibreTexts The 2s orbital would be filled before the 2p orbital because orbitals that are lower in energy are filled first. The 2s orbital is lower in energy than the 2p orbital. There are 5 d orbitals in the d subshell. A p orbital can hold 6 electrons. Based off of the given information, n=4 and ℓ=3. Thus, there are 3 angular nodes present. HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and ... The molecular weight of HCN is 27.025 g/mol. The boiling point of the compound is 78.1 deg F and the melting point is 7.9 deg F. Below are the reactions or methods which lead to the creation of this compound: When methane reacts with ammonia and oxygen we get hydrogen cyanide and water. This reaction is completed when Platinum is added as a ... Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration What is the Orbital Diagram For Nitrogen? When we talk about the orbital diagram, we first need to understand what exactly it means. Therefore, during exams, the student can expect questions related to this topic so the students must go through it. If you are new to such a subject and looking for periodic tables and their other information ...
9.8: Molecular Orbital Theory - Chemistry LibreTexts Molecular Orbital Diagrams This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals.
Which is the molecular orbital diagram for HF? - Quora Answer (1 of 3): The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom. As has been already explained ,only a pz orbital is able to combi...
Oxygen(O) electron configuration and orbital diagram Again, there is only one orbital of the hydrogen atom and it has an electron. The hydrogen atom wants to complete the orbital by receiving an electron. Therefore, two hydrogen atoms share electrons with one oxygen atom to produce water through covalent bonding. Formation of an oxygen compound. One of the elements of group-16 is oxygen.
Molecular Orbital (MO) Diagram of H2 - YouTube Molecular Orbital Diagram for Hydrogen Gas (H2). Fill from the bottom up, with 2 electrons total. Bonding Order is 1, and it is Diamagnetic. sigma2s (2) Check me out:
Orbital Modulation with P Doping Improves Acid and Alkaline Hydrogen ... As a promising green renewable resource, hydrogen energy has been considered as one of the most ideal fuels to solve the energy crisis and environmental pollution [1,2,3].Hydrogen evolution reaction (HER) through electrochemical water splitting has been proved to be an effective and feasible method for hydrogen production [4,5,6].Pt-based nanomaterials are demanded as the best electrocatalyst ...
Orbital diagram - How to draw, Examples, Rules, Filling order There are three rules followed for constructing the orbital diagram. The three rules are - (a). Aufbau's rule (b). Hund's rule (c). Pauli Exclusion rule. (1). Aufbau's principle:- This rule state that the lower energy orbital will be filled before the higher energy orbital, for example - the 1s orbital will fill before the 2s orbital. (2).
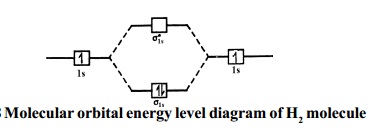
Molecular orbital energy level diagrams -Hydrogen, … The molecular orbital energy level diagram of H2 molecule is given in Fig.. The bond order of H2 molecule can be calculated as follows. Here, Nb = 2 and Na = 0 Bond order = (Nb - Na) /2 = 2-0/2 = 2 i. Nature of bond : This means that the two hydrogen atoms in a molecule of hydrogen are bonded by a single covalent bond. ii.
Why do energy level diagrams differ for hydrogen and ... - Quora You can actually use these energy level diagrams for helium, which (normally) has 2 electrons. What matters is that hydrogen and helium only have one orbital, which can very easily be described. The orbital has no complex functions regarding probability density, which makes the equation different.
H2S Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram ... This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. And on the right-hand side, there will be atomic orbitals of hydrogen. 8 valence electrons are filled in the MO orbitals. There are two non-bonding orbitals present as well.
What is the orbital diagram? - NSN search Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. The Pauli Exclusion Principle says that only two electrons can fit into an single orbital. How do you find the orbital diagram?
Molecular Structure & Bonding - Michigan State University The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond.
Molecular Orbital Theory and Hydrogen Bonding - ScienceMotive 20.11.2020 · Molecular orbitals are formed by the combination of atomic orbitals by an approximate method known as the Linear Combination of Atomic Orbitals (LCAO). According …
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
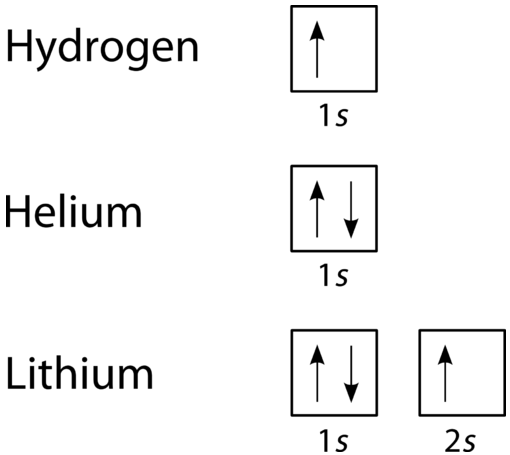
Lithium(Li) electron configuration and orbital diagram So, the electron will enter the 4s orbital first and enter the 3d orbital when the 4s orbital is full. The method of entering electrons into orbitals through the Aufbau principle is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. The first two electrons of lithium enter the 1s orbital. The s-orbital can have a maximum of two electrons.
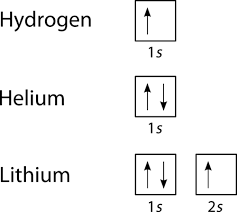
40 Electron Configurations, Orbital Box Notation (M7Q7) - Unizin The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +½).
How to Do Orbital Diagrams | Sciencing Orbital diagrams use the same basic format, but instead of numbers for the electrons, they use ↑ and ↓ arrows, as well as giving each orbital its own line, to represent the spins of the electrons too. Electron Configurations Electron configurations are expressed through a notation that looks like this: 1s 2 2s 2 2p 1.
How to Draw Hybrid Orbital Diagrams for a Molecule by Finding the ... The hybrid orbital diagram of a {eq}sp {/eq} hybridized carbon atom in a carbon dioxide molecule is depicted below. ... There are two hydrogen atoms bonded to the center carbon atom and there are ...
Milankovitch cycles - Wikipedia The semi-major axis of the orbital ellipse, however, remains unchanged; according to perturbation theory, which computes the evolution of the orbit, the semi-major axis is invariant. The orbital period (the length of a sidereal year) is also invariant, because according to Kepler's third law, it is determined by the semi-major axis. Longer-term ...
How do yo write the orbital diagram for hydrogen? | Socratic Orbital diagrams are useful to show the number of electrons, number of electron shells, number of electron pairs, and electron spin directions in a particular atom/ion. Arrows represent electrons, and their spin is represented by which way they point (up or down). Two electrons can be paired into one shell (one little box) as one orbital.
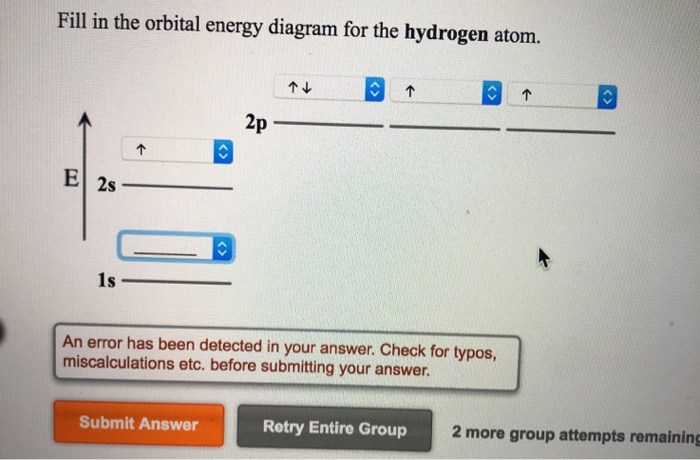
Hydrogen Orbital Diagram - Learnool Here's how you can draw the orbital diagram of hydrogen step by step. Step #1: find electrons of hydrogen Step #2: write electron configuration of hydrogen Step #3: draw orbital diagram of hydrogen Let's break down each step in detail. #1 Find Electrons of Hydrogen The atomic number of hydrogen represents the total number of electrons of hydrogen.
Energy of Orbitals ~ ChemistryGod The energy level diagram for the hydrogen atom. If noticed, the energy gap in successive shells decreases with the energy. The orbitals get closer and closer as we move higher. The electron will mostly spend time in 1s orbital since it is the most stable condition. It can jump to an excited state from the ground state by absorbing energy.
Atomic and Molecular Hydrogen - University of Illinois Urbana-Champaign Below you can see an orbital energy diagram showing the ground state hydrogen atom on the left. When hydrogen absorbs a quantity of energy exactly equal to E 1, the electron goes from the orbital in the first shell (n = 1) to an orbital in the second shell (n = 2). This hydrogen molecule is in an excited state.
2.5.5: Molecular Orbital Diagrams - Chemistry LibreTexts Molecular Orbital Diagrams This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals.
Why do energy level diagrams differ for hydrogen and You can actually use these energy level diagrams for helium, which (normally) has 2 electrons. What matters is that hydrogen and helium only have one orbital, which can very easily be …





















0 Response to "42 orbital diagram for hydrogen"
Post a Comment