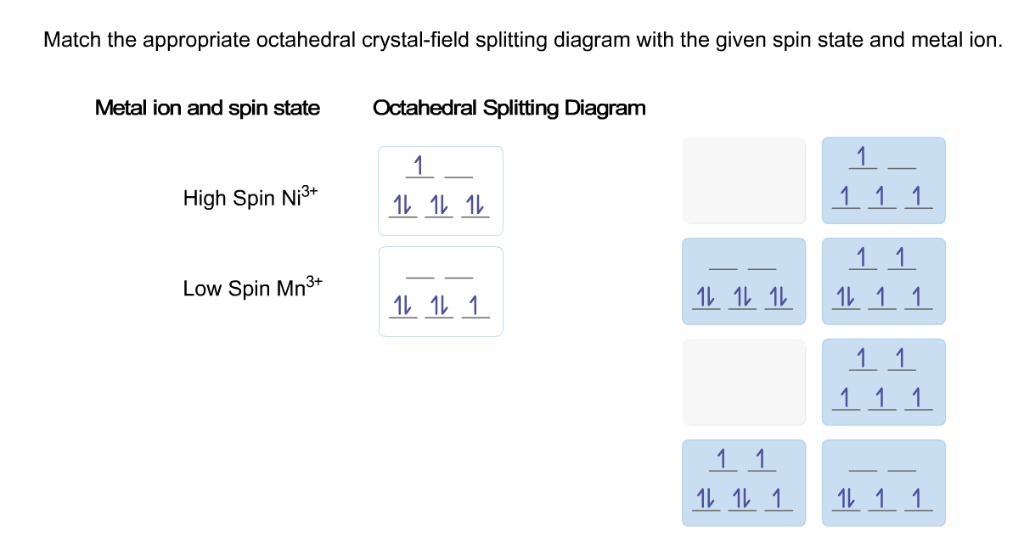
42 match the appropriate octahedral crystal-field splitting diagram ni3+
Solved Match the appropriate octahedral crystal-field - Chegg You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Match the appropriate octahedral crystal-field splitting diagramwith the given spin state and metal ion. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. SOLVED:Match the #ppropriate octahedral crystal - field splitting ... Doctor Octopus Federal crystal splitting field. Chromium three. Plus Looks like that for copper. Two. ... Match the #ppropriate octahedral crystal - field splitting diagram with the given spin stale and metal ion_ Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Co"+ low-spin Colt.
Match the appropriate octahedral crystal-field splitting diagram with ... Draw the octahedral crystal field splitting diagram for each metal ion. Zn^2+ Fe^+ (high- and low-spin) The [Mn(NH_3)_6]^2+ ion is paramagnetic with five unpaired electrons. The NH_3 ligand is usually a strong field ligand.

Match the appropriate octahedral crystal-field splitting diagram ni3+
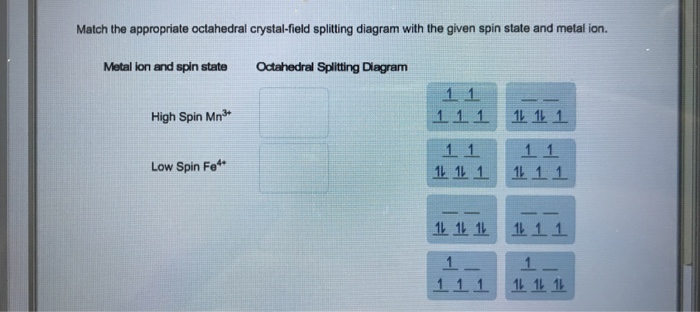
OneClass: Match the appropriate octahedral crystal-field splitting ... Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion Metal ion and spin state Octahedral Splitting Diagram High Spin Mn^2+ Low Spin Mn^2+ Answer + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required. Jarrod Robel Lv2 8 May 2019 Unlock all answers Solved Match the appropriate octahedral crystal-field | Chegg.com Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting Diagram 1L 11 1 High Spin Fe2+ Low Spin Ni3 1L 11 1L 111 This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. OneClass: Match the appropriate octahedral crystal-field splitting ... Get the detailed answer: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ... Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion ...
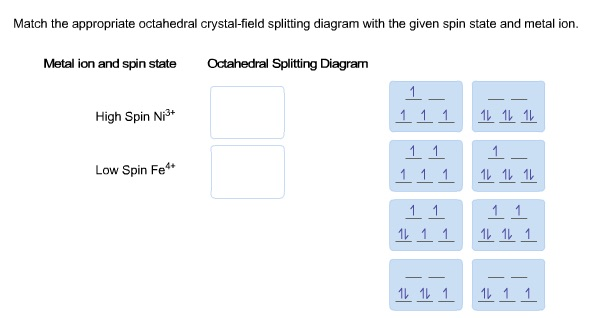
Match the appropriate octahedral crystal-field splitting diagram ni3+. Match the appropriate octahedral crystal-field splitting diagram with ... Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Otahedral Splitting Diagram 4+ High Spin Ni Low Spin Fe Calculate your paper price PDF Crystal Field Splitting in an Octahedral Field - IIT Kanpur Distribution of Electrons in an Octahedral Complex d1 d2 d3 Strong field Weak field Strong field W eak field Strong field Weak field 1 2 Net energy decrease is called crystal field stabilization energy (CFSE) Ford1, CFSE = 1 × 0.4 = 0.4 Δ, CFSE oo For d2, CFSE = 2 × 0.4 = 0.8 Δ o For d3, CFSE = 3 × 0.4 = 1.2 Δ o Solved Match the appropriate octahedral crystal-field - Chegg Ni has 28 electrons and it has the configuration [Ar]3d8 4s2 Ni3+ will have 3 less electrons and its configuration will be [Ar]3d7 … View the full answer Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. 3.8: Bonding in Octahedral Complex Ions- Crystal Field Theory (Crystal field splitting energy also applies to tetrahedral complexes: Δ t.) It is important to note that the splitting of the d orbitals in a crystal field does not change the total energy of the five d orbitals: the two e g orbitals increase in energy by 0.6Δ o, whereas the three t 2g orbitals decrease in energy by 0.4Δ o. Thus the total ...
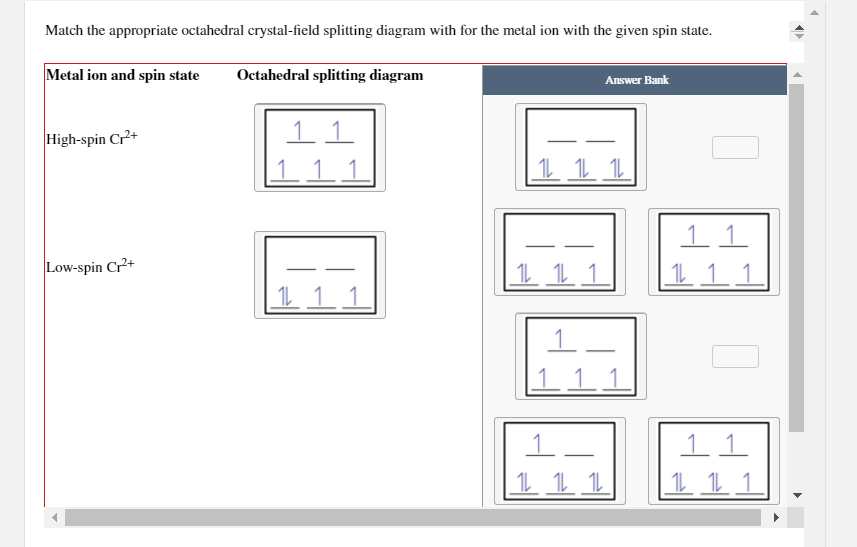
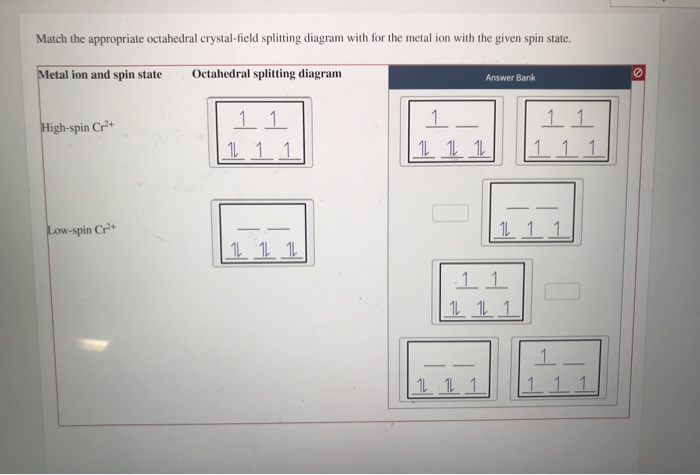
CRYSTAL FIELD SPLITTING IN TETRAHEDRAL COMPLEXES: - Chem Zipper.com In tetrahedral complexes none of the ligand is directly facing any orbital so the splitting is found to be small in comparison to octahedral complexes. For the same metal, the same ligands and metal-ligand distances, it can be shown that del.tetra = (4/9) del.oct. This may attributes to the following two reasons. Solved Match the appropriate octahedral crystal-field - Chegg Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Ni3+ low-spin Cr2+ This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer 24.7: Crystal Field Theory - splitting patterns for octahedral ... The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (Δ o ), where the subscript o stands for octahedral. As we shall see, the magnitude of the splitting depends on the charge on the metal ion, the position of the metal in the periodic table, and the nature of the ligands. Solved Match the appropriate octahedral crystal-field - Chegg Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting Diagram High Spin Ni3+ 1レ 1レ 1レ Low Spin Mn3+ 1レ1レ11レ11 This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
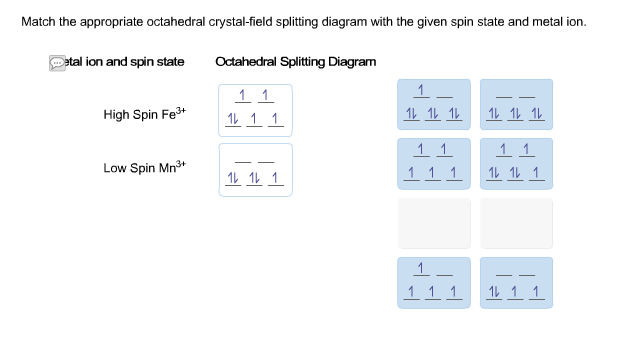
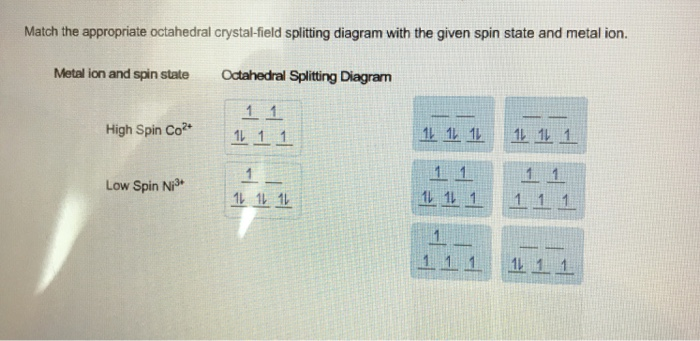
Match the appropriate octahedral crystal-field splitting - Kunduz Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state high-spin Ni³+ low-spin Co³+ Octahedral splitting diagram Show Answer Create an account. Get free access to expert answers Get 3 free question credits Claim your daily rewards Ask your questions by snapping Crystal Field Theory (CFT) - Detailed Explanation with Examples ... - BYJUS The crystal field splitting in a tetrahedral complex is intrinsically smaller in an octahedral filed because there are only two thirds as many ligands and they have a less direct effect of the d orbitals. The relative stabilizing effect of e set will be -6Dq and the destabilizing effect of t 2 set will be +4Dq Crystal Field Stabilization Energy Solved Match the appropriate octahedral crystal‑field - Chegg Question: Match the appropriate octahedral crystal‑field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram high‑spin Fe3+ low‑spin Ni3+ This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer SOLVED: Match the appropriate octahedral crystal-field splitting ... SOLVED: Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state Metal ion and spin state Octahedral splitting diagram Answer Bank HHigh-spin Co3+ 1 LLow-spin CoS+ 11i 11 VIDEO ANSWER:Hi in this question, we have to draw the octahedron splitting diagram of FE three plus.
Crystal field theory - Wikipedia If the splitting of the d-orbitals in an octahedral field is Δ oct, the three t 2g orbitals are stabilized relative to the barycenter by 2 / 5 Δ oct, and the e g orbitals are destabilized by 3 / 5 Δ oct.As examples, consider the two d 5 configurations shown further up the page. The low-spin (top) example has five electrons in the t 2g orbitals, so the total CFSE is 5 x 2 / 5 Δ oct = 2Δ oct.
11.1: Introduction to Crystal Field Theory (Octahdral complexes) The energy of an electron in any of these three orbitals is lower than the energy for a spherical distribution of negative charge. Figure 11.1. 1: An Octahedral Arrangement of Six Negative Charges around a Metal Ion Causes the Five d Orbitals to Split into Two Sets with Different Energies. (a) According to CFT, the uniform distribution of ...
OneClass: Match the appropriate octahedral crystal-field splitting ... Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High Spin Mn^3 + Low Spin Ni^3 Answer + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required. Irving Heathcote Lv2 25 Jan 2019 Unlock all answers Get 1 free homework help answer. Unlock Already have an account?
Crystal Field Splitting - an overview | ScienceDirect Topics This crystal field splitting has been observed for the methylene rocking mode at 720 cm −1 and for the methylene bending mode at 1460 cm −1 in spectra of crystalline PE. Although other modes should also exhibit such splitting, their inherent bandwidth prevents the observation of separate components. When PE is melted, the crystal field splitting disappears.
Solved Match the appropriate octahedral crystal‑field - Chegg Match the appropriate octahedral crystal‑field splitting diagram with the given spin state and metal ion. high-spin Co2+ low-spin Mn3+ Show transcribed image text Expert Answer Please like t … View the full answer Transcribed image text: Maich the appropriate octahedral crystal-field splitting diagram with the given spin stato and metal ion.
Crystal Field Theory | CFT | Crystal Field Splitting in Octahedral ... It is clear that CN 1-ligand produces more splitting and hence it is a strong ligand while Cl 1-ligand produces less splitting and hence is a weak ligand.. Charges on the Metal; The magnitude of D 0 increases as the charge on the metal ions increases. A metal ion with a higher charge draws the ligands closer, and hence produces more splitting than an ion with a lower charge. e.g.
Crystal Field Theory - Chemistry LibreTexts Here it is an octahedral which means the energy splitting should look like: Step 3: Determine whether the ligand induces is a strong or weak field spin by looking at the spectrochemical series. Cl - is a weak field ligand (i.e., it induces high spin complexes). Therefore, electrons fill all orbitals before being paired.
SOLVED:Draw the octahedral crystal field splitting diagram ... - Numerade Draw the octahedral crystal field splitting diagram for each metal ion. a. Zn2+ b. Fe3+ (high- and low-spin) c. V3+ d. Co2+ (high-spin) Answer A) The octahedral crystal field splitting diagram for the given metal ion is as follows: IMAGE NOT AVAILABLE B) The octahedral crystal field splitting diagram for F e 3 + in low spin complex is as follows:
Match the appropriate octahedral crystal-field | Chegg.com Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Ni3+ low-spin Fe4+ Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion.
Crystal Field Theory - Purdue University Octahedral Crystal Fields Each Mn 2+ ion in manganese (II) oxide is surrounded by six O 2- ions arranged toward the corners of an octahedron, as shown in the figure below. MnO is therefore a model for an octahedral complex in which a transition-metal ion is coordinated to six ligands.
OneClass: Match the appropriate octahedral crystal-field splitting ... Get the detailed answer: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ... Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion ...
Solved Match the appropriate octahedral crystal-field | Chegg.com Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting Diagram 1L 11 1 High Spin Fe2+ Low Spin Ni3 1L 11 1L 111 This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
OneClass: Match the appropriate octahedral crystal-field splitting ... Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion Metal ion and spin state Octahedral Splitting Diagram High Spin Mn^2+ Low Spin Mn^2+ Answer + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required. Jarrod Robel Lv2 8 May 2019 Unlock all answers


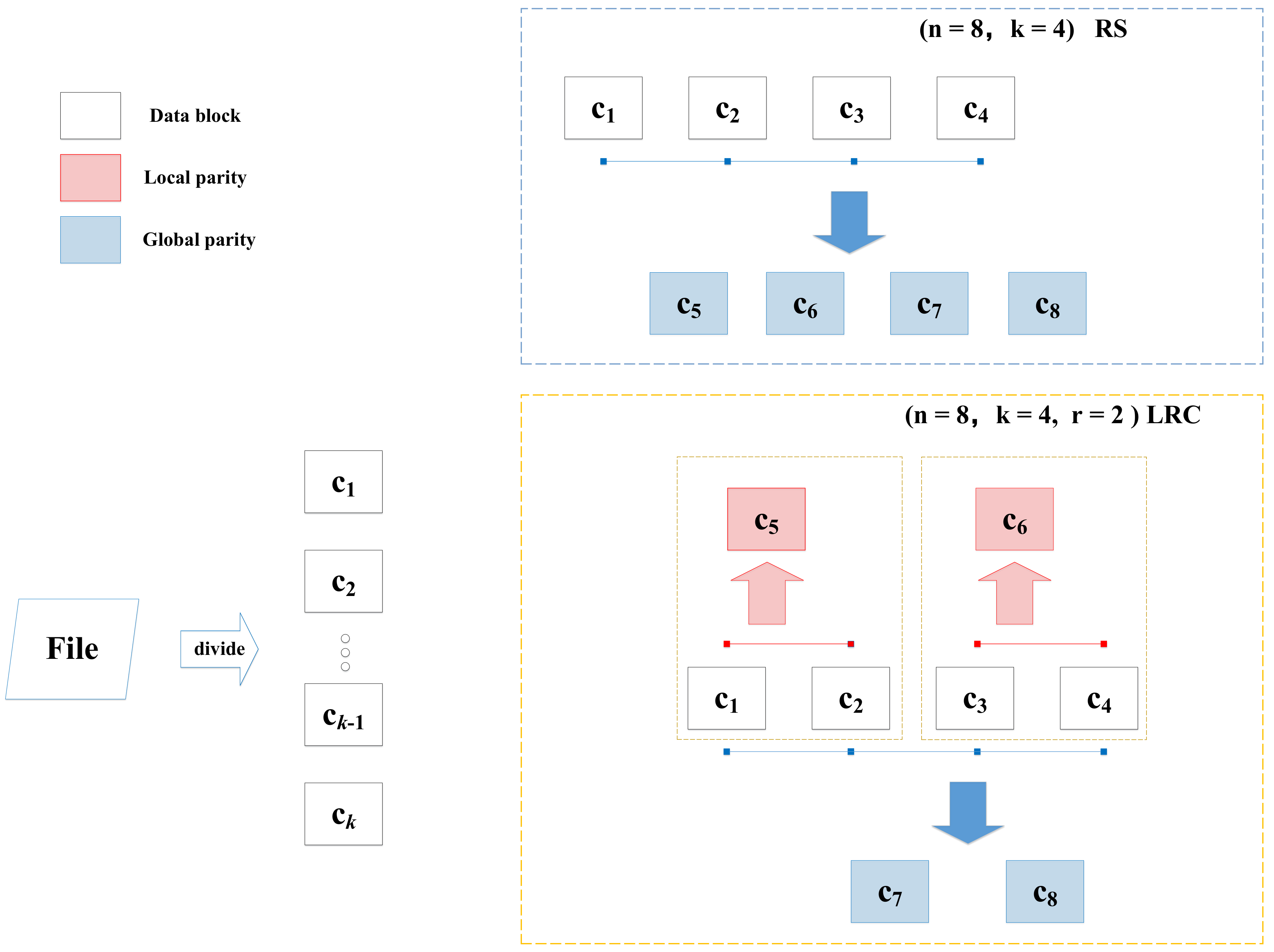

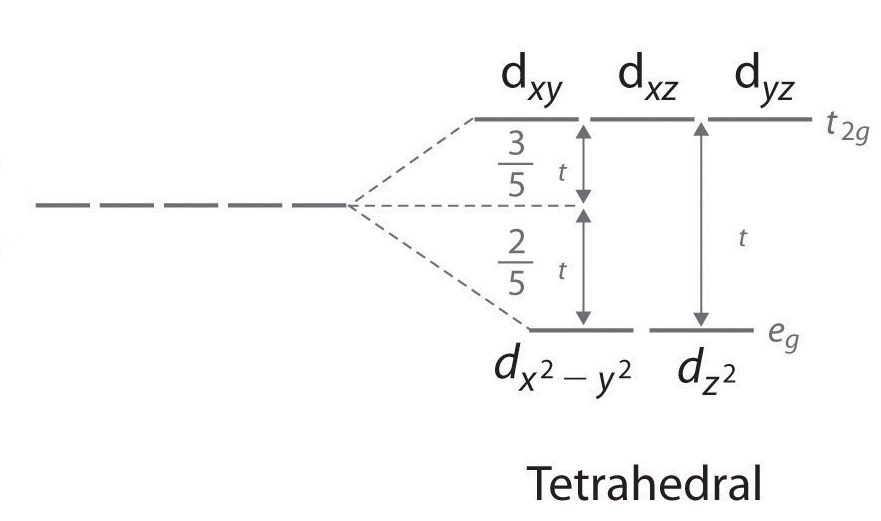
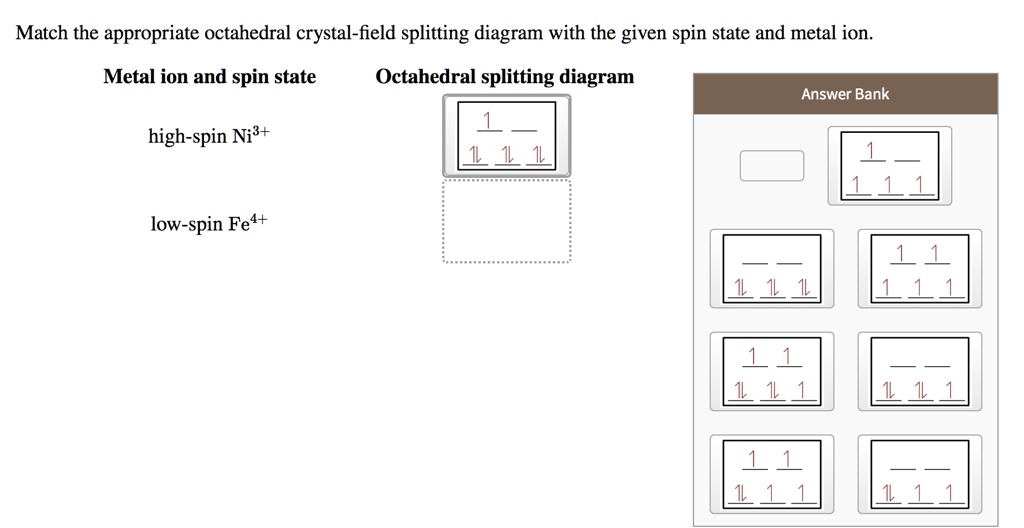



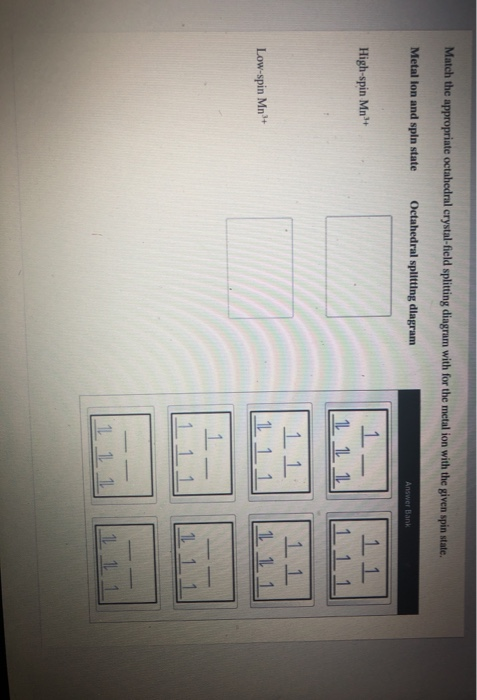






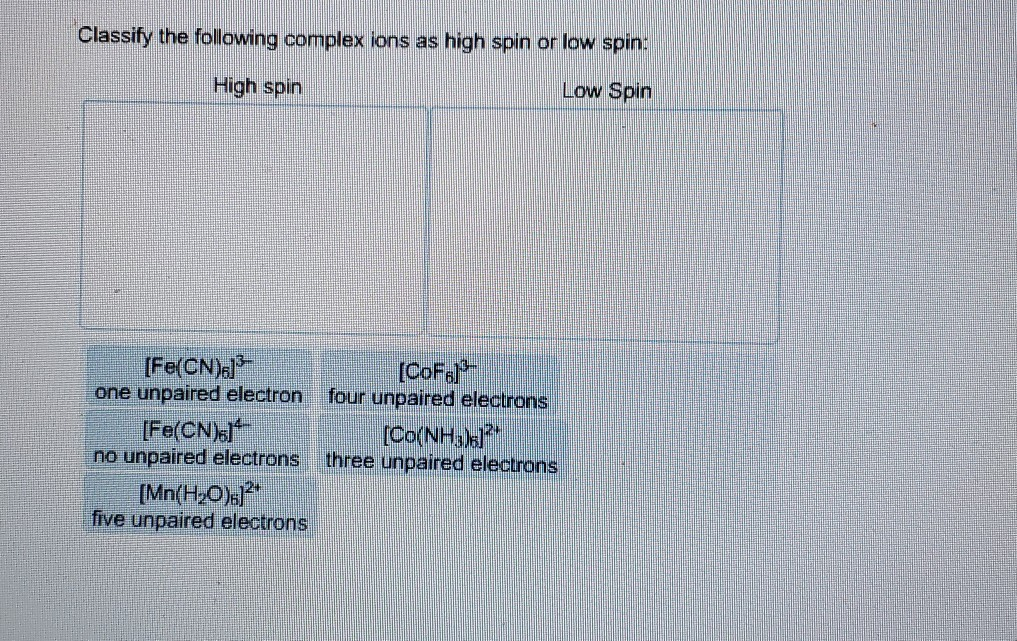

0 Response to "42 match the appropriate octahedral crystal-field splitting diagram ni3+"
Post a Comment