41 octahedral crystal field splitting diagram
SOLVED:Draw the octahedral crystal field splitting diagram ... - Numerade Draw the octahedral crystal field splitting diagram for each metal ion. a. Zn2+ b. Fe3+ (high- and low-spin) c. V3+ d. Co2+ (high-spin) Answer A) The octahedral crystal field splitting diagram for the given metal ion is as follows: IMAGE NOT AVAILABLE B) The octahedral crystal field splitting diagram for F e 3 + in low spin complex is as follows: Crystal Field Theory (CFT) - Detailed Explanation with Examples & Videos The splitting in various crystal fields is discussed below: Crystal Field Splitting in Octahedral Complex In the case of an octahedral coordination compound having six ligands surrounding the metal atom/ion, we observe repulsion between the electrons in d orbitals and ligand electrons.
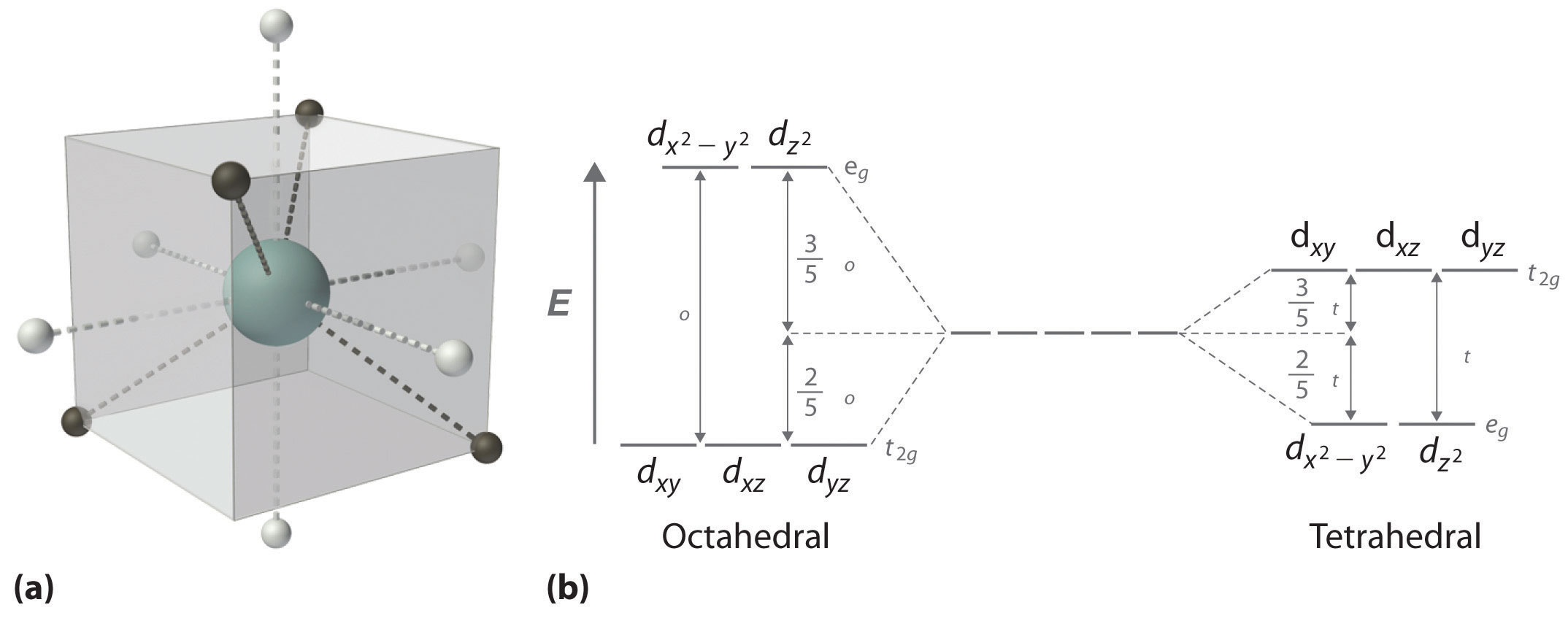
Crystal field theory - Wikipedia The crystal field splitting energy for tetrahedral metal complexes (four ligands) is referred to as Δ tet, and is roughly equal to 4/9Δ oct (for the same metal and same ligands). Therefore, the energy required to pair two electrons is typically higher than the energy required for placing electrons in the higher energy orbitals.

Octahedral crystal field splitting diagram
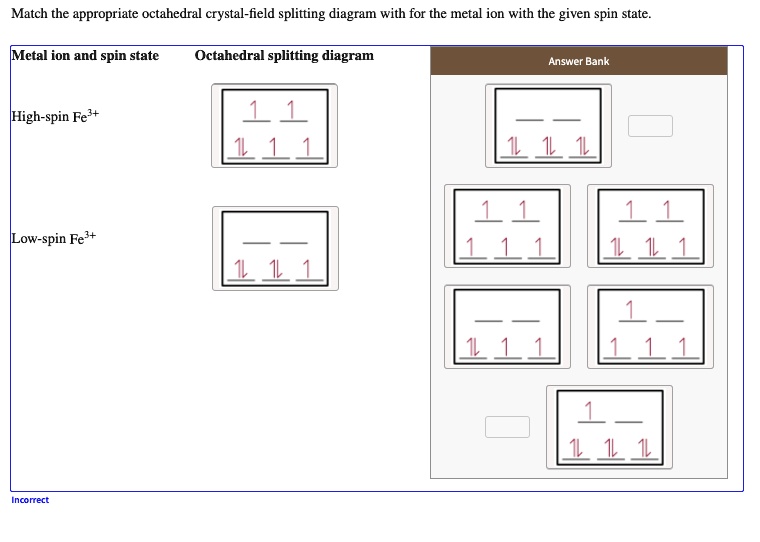
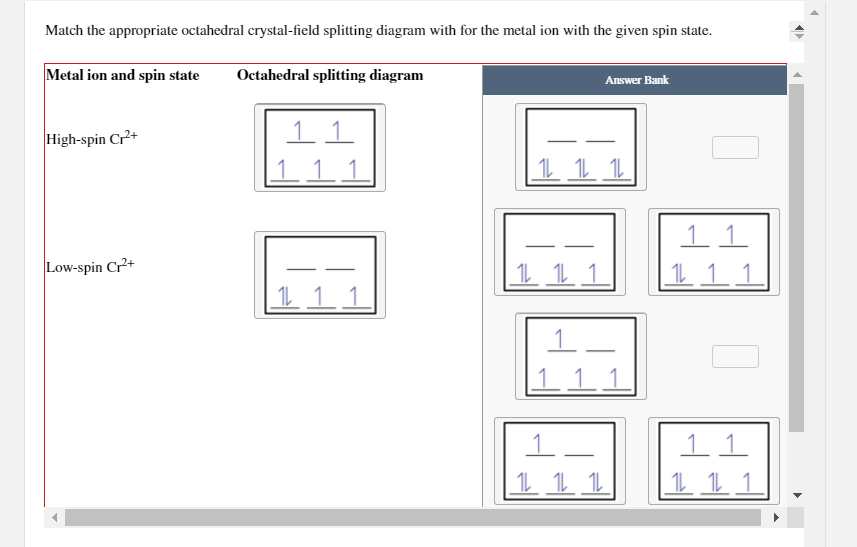
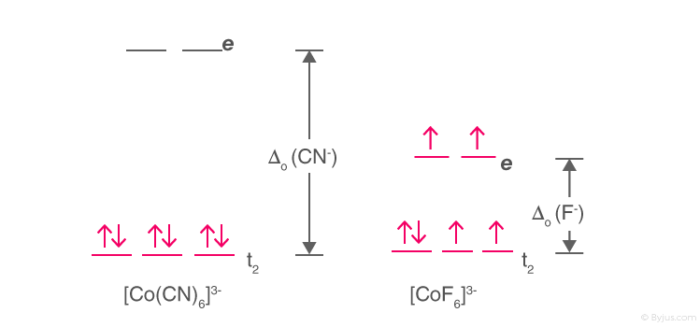
Crystal field splitting for the octahedral coordinated Co +3-ions and ... View publication Crystal field splitting for the octahedral coordinated Co +3-ions and the tetrahedral coordinated Co +3-ions. Source publication Investigations on RF-magnetron sputtered Co 3... Match the appropriate octahedral crystal-field splitt… - SolvedLib Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion; Metal ion and spin state ... Crystal field theory and not cathedral field splitting diagrams. And to review uh we can split our five D orbital's shown here in the graphics into two groups, the two G and the E. G orbital sets, which are here ... SOLVED: Match the appropriate octahedral crystal-field splitting ... Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state Metal ion and spin state Octahedral splitting diagram Answer Bank HHigh-spin Co3+ 1 LLow-spin CoS+ 11i 11
Octahedral crystal field splitting diagram. CRYSTAL FIELD SPLITTING IN TETRAHEDRAL COMPLEXES: - Chem Zipper.com (1) There are only four ligands instead of six, so the ligand field is only two thirds the size; as the ligand field spliting is also the two thirds the size and (2) The direction of the orbitals does not concide with the direction of the ligands. This reduces the crystal field spliting by roughly further two third. Tanabe–Sugano diagram - Wikipedia WebBackground. Until Yukito Tanabe and Satoru Sugano published their paper "On the absorption spectra of complex ions", in 1954, little was known about the excited electronic states of complex metal ions.They used Hans Bethe's crystal field theory and Giulio Racah's linear combinations of Slater integrals, now called Racah parameters, to explain … Browse Articles | Nature Materials Web08.12.2022 · The authors fabricate a fluxonium circuit using a granular aluminium nanoconstriction to replace the conventional superconductor–insulator–superconductor tunnel junction. Spin-polarized oxygen evolution reaction under magnetic field Web10.05.2021 · The oxygen evolution reaction (OER) is the bottleneck that limits the energy efficiency of water-splitting. The process involves four electrons’ transfer and the generation of triplet state O2 ...
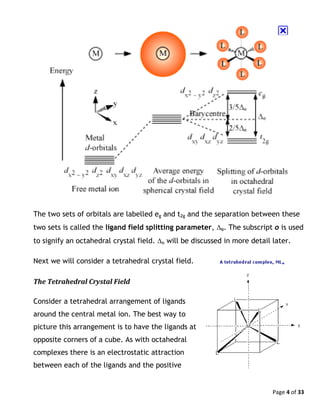
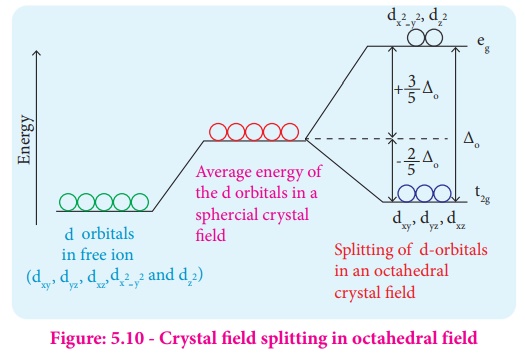
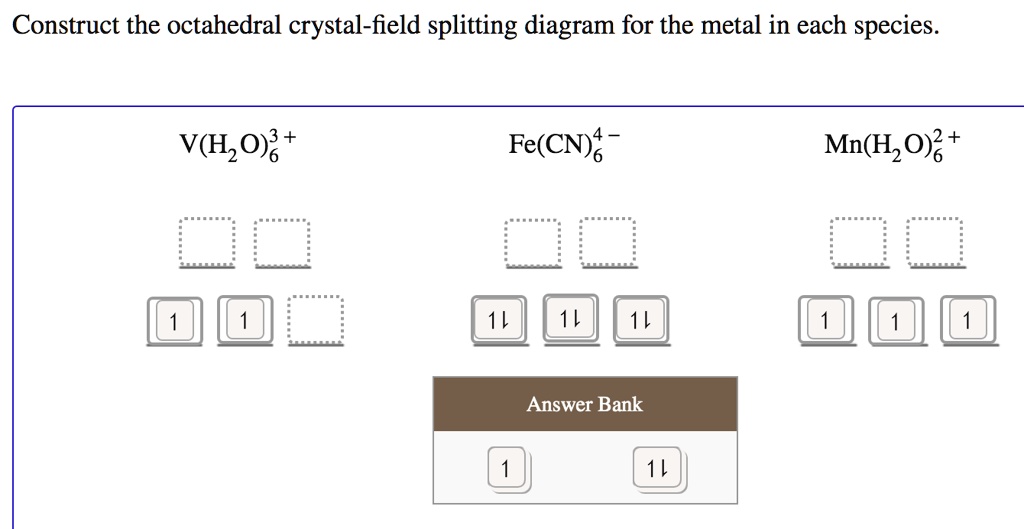
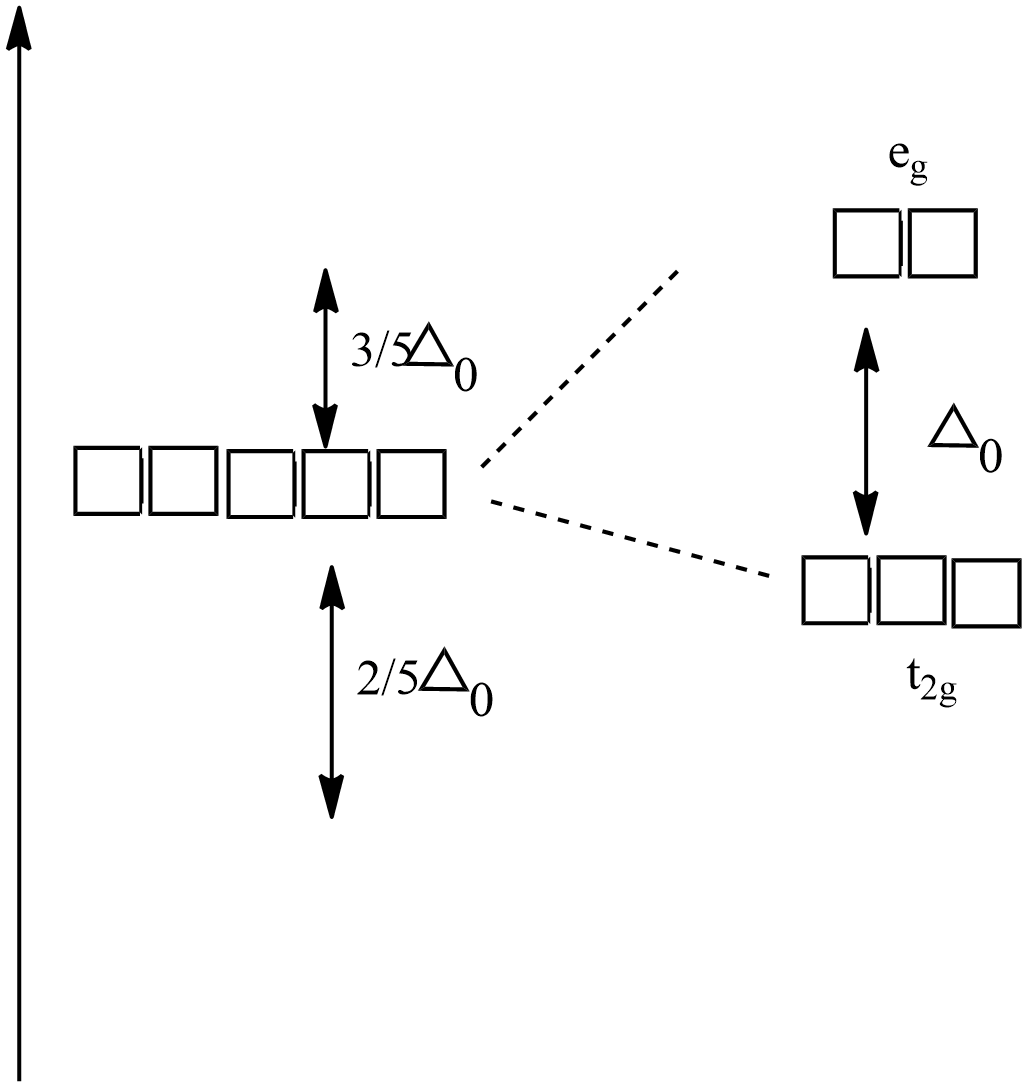
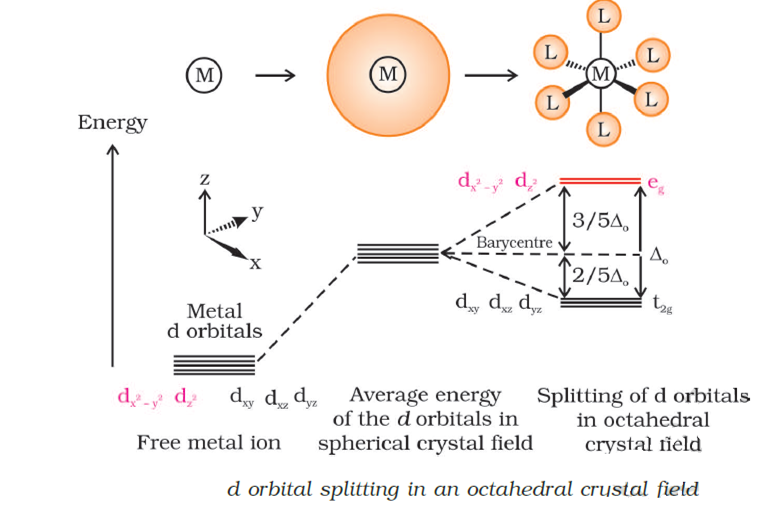
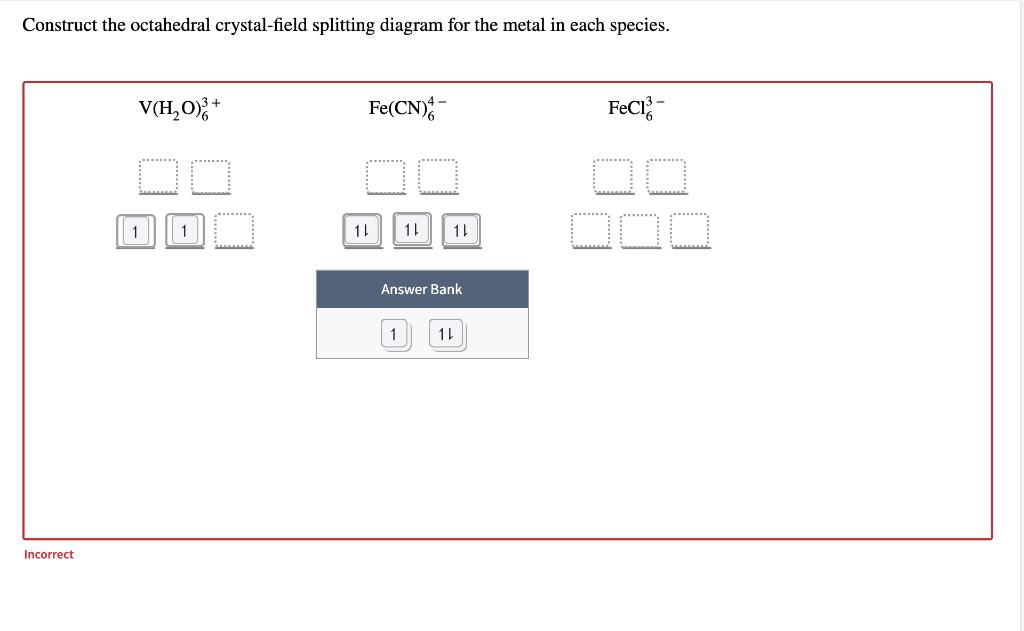
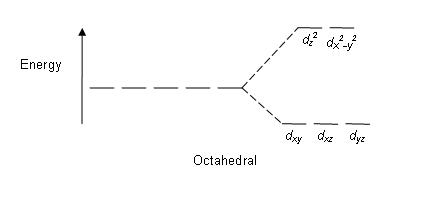
Octahedral Crystal Field Splitting Orbital Degeneracy The crystal field splitting is based on where the ligands (modelled as point charges) are in relation to the orbitals. In an octahedral complex, the ligands are all at 90° from each other and are placed on each of the x, y, z axes. The orbitals that lie on these axes will experience the most repulsion and will rise in energy, while the ... PDF Crystal Field Splitting in an Octahedral Field - IIT Kanpur Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg o. Molecular Orbital Diagram || 3d Series Metals || Octahedral Geometry ... CONNECT WITH TEAM CHEMISTRY UNTOLD :- CHANNEL MEMBERSHIP :- untold face book link :- h... Solved Construct the octahedral crystal-field splitting - Chegg Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H20)3+ Co (CN) - FeCl3 - DO ODO OO CO DO OOO Answer Bank 1 11 Question: Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H20)3+ Co (CN) - FeCl3 - DO ODO OO CO DO OOO Answer Bank 1 11 This problem has been solved!
Join LiveJournal WebPassword requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; For the crystal field splitting in octahedral complexes? What is the correct splitting pattern for an octahedral complex? For octahedral complexes, crystal field splitting is denoted by Δo (or Δoct). The energies of the dz2 and dx2−y2 orbitals increase due to greater interactions with the ligands. The dxy, dxz, and dyz orbitals decrease with respect to this normal energy level and become more stable. Splitting d orbitals in an octahedral - Big Chemical Encyclopedia A low-spin to high-spin transition relates to the crystal field splitting of the d-orbitals in an octahedral or tetrahedral crystal field. However, even in cases where the energy difference between two spin states is much larger, electronic transitions are observed. An atom with total spin quantum number S has (22 + 1) orientations. PDF Crystal Field Splitting in Octahedral Transition Metal Complexes - umb.edu d‐Subshell Splitting in an O h Field • In the octahedral (O h) environment of three acac ligands, the fivefold degeneracy among the d orbitals in Mn3+ islifted. • To a first approximation, the ligand field is of O h symmetry, and the 3 d orbitals will separate into a set of three degenerate orbitals (t 2g = dxy, dyz, dxz) and a set of two
Octahedral crystal field energy level diagram - Big Chemical Encyclopedia Figure 3.7 Simplified energy level diagram for 3d6 ions (e.g., Fe2+ and Co3+) in an octahedral crystal field. The diagram shows that in a high intensity field the 1Alg crystal field state, corresponding to the low-spin configuration (t2gf, becomes the ground state. Figure 3.8 Tanabe-Sugano energy level diagram for a 3d6 ion in an octahedral ...
JoVE | Peer Reviewed Scientific Video Journal - Methods and Protocols The difference in energy between the e g and the t 2g orbitals is called the crystal field splitting and is symbolized by Δ oct, where oct stands for octahedral. The magnitude of Δ oct depends on many factors, including the nature of the six ligands located around the central metal ion, the charge on the metal, and whether the metal is using ...
What is the crystal field splitting of an octahedral complex? In simple words , in Crystal field splitting there is a splitting of d orbitals into t2g and eg energy levels with respect to ligands interaction with these orbitals. For octahedral complex , there is six ligands attached to central metal ion , we understand it by following diagram of d orbitals in xyz plane.
Difference Between Octahedral And Tetrahedral Complexes Octahedral complexes have six ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. Octahedral molecular geometry describes the shape of compounds wherein six atoms or groups of atoms or ligands are symmetrically arranged around a central atom. The octahedron has eight faces, hence the prefix octa-.
Phase patterning for ohmic homojunction contact in MoTe2 Aug 07, 2015 · In contrast to 2H-MoTe 2, in which two Te atoms completely overlap in the top view of the crystal in the STEM images, 1T'-MoTe 2 should show split Te positions in the top view STEM image . The initial stage of splitting the Te atom positions is captured in Fig. 4D, which is a filtered STEM image to clearly show the Te atoms. As marked by two ...
Ionic compound - Wikipedia WebIn chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding.The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions.These can be simple ions such as the sodium (Na +) and chloride (Cl −) in sodium chloride, or …
Octahedral Crystal-field Splitting Diagram For V(h2o)63+ - ICASMT The difference in energy between the e g and the t 2g orbitals is called the crystal field splitting and is symbolized by Δoct, where oct stands for octahedral. The magnitude of Δ oct depends on many factors, including the nature of the six ligands located around the central metal ion, the charge on the metal, and whether the metal is using 3.
Crystal Field Splitting - an overview | ScienceDirect Topics Crystal field d orbital splitting diagrams for common geometries. The above treatment considers the ligands in an octahedral geometry (i.e., with the ligands placed at the centre of the faces of the cube). The square planar case is simply a special case of the octahedral symmetry where two ligands are removed from the z -axis.
Crystal Field Splitting-Octahedral, Tetrahedral and Square Planar Watch Crystal Field Splitting-Octahedral, Tetrahedral and Square Planar in English from Crystal Field Theory here. Watch all CBSE Class 5 to 12 Video Lectures here.
Two examples of crystal field splitting. (a) octahedral (b ... Download scientific diagram | Two examples of crystal field splitting. (a) octahedral (b) orthorhombic. from publication: Crystal-field effects in graphene with interface-induced spin-orbit ...
Solved Construct the octahedral crystal-field splitting - Chegg Question: Construct the octahedral crystal-field splitting diagram for the metal in each species. V ( H 2 O ) 3 + 6 V (H2O)63+ Co ( CN ) 3 − 6 Co (CN)63− Mn ( H 2 O ) 2 + 6 Mn (H2O)62+ Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)6 (3+)charge Co (CN)6 3− Mn (H2O)6 2+ This problem has been solved!
Draw figure to show the splitting of d orbitals in an octahedral ... The crystal field splitting energy for octahedral and tetrahedral complexes is related as Q. Low spin complex of d6− cation in an octahedral field will have the following energy: (Δ0 = crystal feild splitting energy in an octahedral feild, P= Electron pairing energy)
Crystal Field Theory - Chemistry LibreTexts Web06.05.2021 · There is a large energy separation between the d z² orbital and the d xz and d yz orbitals, meaning that the crystal field splitting energy is large. We find that the square planar complexes have the greatest crystal field splitting energy compared to all the other complexes. This means that most square planar complexes are low spin, strong ...
24.7: Crystal Field Theory - splitting patterns for octahedral ... The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (Δ o ), where the subscript o stands for octahedral. As we shall see, the magnitude of the splitting depends on the charge on the metal ion, the position of the metal in the periodic table, and the nature of the ligands.
Crystal Field Theory - Purdue University Crystal field theory was developed by considering two compounds: manganese (II) oxide, MnO, and copper (I) chloride, CuCl. Octahedral Crystal Fields Each Mn 2+ ion in manganese (II) oxide is surrounded by six O 2- ions arranged toward the corners of an octahedron, as shown in the figure below.
Crystal Field Theory | CFT | Crystal Field Splitting in Octahedral ... Explain in brief crystal field splitting in the octahedral complexes. In an octahedral complex, the metal ion is at the center of the regular octahedron and ligands are at the six corners of the octahedron along the X, Y, and Z axes. In free metal ion, all the five d-orbitals have the same energy i.e. they are degenerate (State-I).
Crystal Field Theory - YouTube Crystal Field Theory 184,499 views Jan 2, 2021 This chemistry video tutorial provides a basic introduction into crystal field theory. It explains how to draw the crystal field splitting...
Improving the oxygen redox reversibility of Li-rich battery cathode ... Web02.03.2022 · Herein, we reveal that the enhancement of oxygen redox reversibility and the mitigation of TM migration in Li-rich Mn-based oxide (Li 1.2 Mn 0.6 Ni 0.2 O 2) materials with oxygen vacancies are ...
Metal ions in aqueous solution - Wikipedia Values for transition metals are affected by crystal field stabilization. The general trend is shown by the magenta line which passes through Ca 2+, Mn 2+ and Zn 2+, for which there is no stabilization in an octahedral crystal field. Hydration energy increases as size decreases. Crystal field splitting confers extra stability on the aqua ion.
Solid solution - Wikipedia WebNomenclature. The IUPAC definition of a solid solution is a "solid in which components are compatible and form a unique phases".. The definition "crystal containing a second constituent which fits into and is distributed in the lattice of the host crystal" given in refs., is not general and, thus, is not recommended. The expression is to be used to describe a …
SOLVED: Match the appropriate octahedral crystal-field splitting ... Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state Metal ion and spin state Octahedral splitting diagram Answer Bank HHigh-spin Co3+ 1 LLow-spin CoS+ 11i 11
Match the appropriate octahedral crystal-field splitt… - SolvedLib Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion; Metal ion and spin state ... Crystal field theory and not cathedral field splitting diagrams. And to review uh we can split our five D orbital's shown here in the graphics into two groups, the two G and the E. G orbital sets, which are here ...
Crystal field splitting for the octahedral coordinated Co +3-ions and ... View publication Crystal field splitting for the octahedral coordinated Co +3-ions and the tetrahedral coordinated Co +3-ions. Source publication Investigations on RF-magnetron sputtered Co 3...






















0 Response to "41 octahedral crystal field splitting diagram"
Post a Comment